


Reactivity of red phosphorus
At room temperature, red phosphorus is quite inert, and it is non-toxic, due to this inertness. Also, there is no solvent for red phosphorus. It either reacts, or it is insoluble, in every known solvent.
At elevated temperatures, however, red phosphorus is extremely reactive, much more so than sulphur or carbon. When it is mixed with an oxidizer, then the mix is very sensitive, either for friction, for heating, or for both.
 In
this experiment, a mix of red phosphorus and potassium periodate is made. This
mix, when ignited, explodes, even when not confined. When a quantity of
over approximately 10 mg of the mix is ignited, then a loud BANG is produced,
with smaller quantities of around 5 mg, a bright purple/white flash, and a loud
WHOOP noise is produced. Do not ignite quantities over 25 mg of this mix.
That may lead to serious injury.
In
this experiment, a mix of red phosphorus and potassium periodate is made. This
mix, when ignited, explodes, even when not confined. When a quantity of
over approximately 10 mg of the mix is ignited, then a loud BANG is produced,
with smaller quantities of around 5 mg, a bright purple/white flash, and a loud
WHOOP noise is produced. Do not ignite quantities over 25 mg of this mix.
That may lead to serious injury.
![]()
![]() Required
chemicals:
Required
chemicals:
-
red phosphorus
-
potassium periodate (potassium perchlorate can be used also and gives very similar results,
 do not use potassium chlorate or potassium bromate instead)
do not use potassium chlorate or potassium bromate instead)
![]() Required
equipment:
Required
equipment:
-
small oil lamp or alcohol lamp
-
metal spatula
-
weighing equipment, capable of weighing at 1 mg resolution
![]() Safety:
Safety:
-
 Only mix VERY small quantities. Only the very small amount, used in this
experiment should be mixed, and no mix should be stored.
Only mix VERY small quantities. Only the very small amount, used in this
experiment should be mixed, and no mix should be stored. -
When preparing the mix, be very cautious in mixing the two powders. Too much friction and static discharge may result in premature setting off of the mix, which may lead to serious injury.
-
 When the chemicals are crystalline or clumpy, first grind them separately.
After grinding, the fine powders can be mixed.
When the chemicals are crystalline or clumpy, first grind them separately.
After grinding, the fine powders can be mixed.
![]() Disposal:
Disposal:
-
The only waste, produced in this experiment is the smoke. This smoke is somewhat toxic due to its iodine content, but the amount, produced in this experiment is so small, that the toxicity is negligible. The spatula, holding the exploding mix should be rinsed and cleaned thoroughly after the experiment.
![]()
Preparation of the mix of red phosphorus and KIO4
![]() Take
approximately 20 mg of finely powdered potassium periodate.
Take
approximately 20 mg of finely powdered potassium periodate.
![]() Take approximately 5 mg of finely powdered red phosphorus.
Take approximately 5 mg of finely powdered red phosphorus.
These quantities are very small and it may be difficult to weigh out such small quantities. But, if you are not capable of weighing out such small amounts, then do not weight out larger amounts! An explosion of e.g. 100 mg of mix may result in serious material damage and bad burns, but it may also damage your ears, especially, when the explosion occurs in a small room. If you cannot weight out small amounts precisely, then simply estimate the amount of power with your eyes. Take roughly 4 mm≥ or so of red phosphorus and 10 mm≥ of powdered potassium periodate and mix that.
Put both solids on a small piece of paper, folded in such a way that the solid does not easily fall off the paper. Carefully mix the two solids by swirling around the paper. Do not use a stick or spatula to mix the chemicals. Friction or static discharge may result in premature ignition.
Performing the experiment
Take a small metal spatula, and put a pile of the mix on the spatula. Do this with great care. The pile of powder should have a size of approximately 2.5x2.5 mm≤, and the top of the heap may be approximately 2 mm in height. The total volume of powder is estimated to be between 5 and 10 mm≥.
The spatula slowly is moved towards a flame. When the spatula enters the heat of the air stream of the flame, then the mix explodes with a deep WHOOP sound and a very bright purple/white flash. The effect is very fast, it all takes less time then the blink of an eye. A digital camera was used to record the explosion at a frame rate of 60 frames per second. The lighting conditions were aperture f/2.8, exposure time 1/50th second for a well exposed negative, if a standard 24x36 mm negative film were used at 50 ASA.
Below, 9 frames are shown of the explosion and the subsequent cloud of smoke.









The sequence shows that the smoke is somewhat pink/purple. This is due to the formation of some free iodine in this experiment. The amount of iodine, however, is very small. After the experiment, there is no noticeable smell of iodine, but apparently there is sufficient for giving a purple tinge to the smoke. The third frame is completely overexposed. The flash is very bright and the camera was not capable of handling that extreme light in a proper way. It is remarkable how short the flash lasts. Each frame represents only 1/60th of a second. All 9 frames above only cover 0.15 second.
Most remarkable is the second frame. This is the onset to explosion. It looks as if the solid is literally blown away from the spatula and that the real explosion is in the air, instead of on the spatula. The third frame, however, does not yield any more information, so this set of frames is not conclusive. Additional experiments have been performed, but all show similar results, with the frame of the explosion itself completely overexposed. In all experiments, the bright explosion always takes 1 frame.
Here is a little slow-motion movie, which shows the explosion at ⅓ of the real-time speed. Size is approximately 150 Kbyte, download speed is limited to 50 Kbyte/s. Even in slow-motion, the effect is very fast and is hard to observe.
A second experiment, emphasis on smoke cloud
In the following sequence, a more wide-angle setting was used at the camera, such that the smoke could be better observed. This sequence also has an overexposed image. The effect at the onset of explosion now is not observed, it probably happened in the same timeframe as where the large shot of light is produced, so it drowns in the overexposure. But now, a nice effect after explosion can be observed. The cloud of smoke is glowing, giving orange light. This afterglow also is very short-lasting. The next frame, the glow is gone.









Effect of using free flowing powder
In this final experiment the effect of throwing the free flowing powder in the flame of the burner is studied. So, instead of putting a pile of 6 mg on a spatula, approximately 6 mg of powder is thrown into a flame. Even with the free powder, an explosion occurs. But, the effect is slightly longer-lasting. Because of spreading of the powder, the flash needs a little more time to spread throughout the entire powder. There also is a longer burning after the initial explosion. This is due to small particles, which are lagging behind the main cloud of powder. These small particles ignite slightly later. But still, the effect is very fast and within 200 ms everything is over again. Below follow six frames, with the powder falling down.






These 6 pictures nicely show the powder falling down. They also show that the cloud of powder is dispersed over a larger volume, while falling down. There is a tail of particles, lagging behind the main cloud of powder.
Then, at once, while there still is quite some distance from the flame, the powder ignites. The camera is totally wreaking havoc when this occurs. Unfortunately, no detail is shown at all. After 33 ms, when the initial explosion is over, a glowing cloud of smoke can be observed. This cloud of smoke still is very hot. It emits orange light.



The next pictures show what happens with the glowing cloud of smoke. It contains lots of burning particles and in spite of its weight, it is moving upwards again, due to the heating up of the air. Again, the purple color, due to formation of small amounts of iodine, can be observed very nicely.
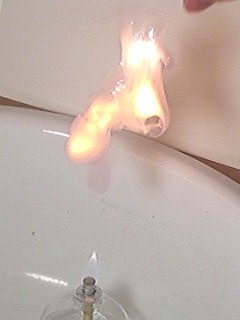
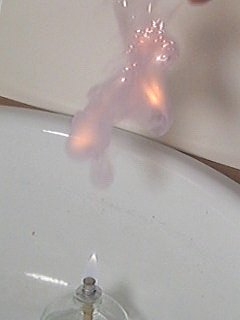







Again, a small movie is available which shows this experiment in slow-motion at ⅓ of real-time speed. The movie can be downloaded here. Size is approximately 150 Kbyte, download speed is limited to 50 Kbyte/s.
All these experiments show that red phosphorus reacts violently with potassium periodate and that only limited heat is needed for that. They also show, how dangerous mixtures of red phosphorus with an oxidizer like potassium periodate are. Even with the very small quantities used here, the effect already is very impressive. With larger quantities, accidents certainly will occur, unless the experiment is performed outside, and a remote ignition mechanism is used.
![]()
Discussion of results
Red phosphorus is a strong reductor at elevated temperatures and it is very reactive in those situations.
The oxidizer, used here, has a large amount of available oxygen, which binds to the phosphorus to form the highest oxide. The main reaction is:
5KIO4(s) + 8P → 5KI + 2P4O10
There also are side reactions, most likely a small amount of lower oxide P4O6 is formed. Also some free iodine is formed.
![]()
Remark: All experiments, shown above, can also be performed with KClO4, instead of KIO4. Instead of 20 mg, then approximately 12 mg should be taken for the same amount of red phosphorus. The flash looks the same, the smoke is purely white when KClO4 is used.
I also attempted to do the experiment with KClO3 and KBrO3. Both of these are unfit for performing this kind of experiments. KClO3 and red phosphorus cannot be mixed safely. On one occasion, the mix ignited, while mixing very gently. The chemical KBrO3 cannot be mixed with red phosphorus at all. This always results in explosion while mixing!