A polyhalide salt of potassium
It is known that the halogens form interhalogen compounds. An example of two interhalogen compounds is shown in this experiment, where iodine and chlorine react to form iodine chlorides.
A similar phenomenon also exists for halide ions, which can combine with halogens to form so-called polyhalide ions. The most common one is I3, formed on dissolving I2 in a concentrated solution of KI. Solid salts can be isolated of these polyhalide ions. E.g. solid KI3 can be obtained from a very concentrated solution of KI/I2. These polyhalide salts unfortunately are very unstable and it is not worth the effort to isolate them for long-term storage. They decompose very easily.
It is nice, however, to prepare such polyhalide salts just as curiosities. In this experiment a much less known polyhalide salt is prepared: potassium tetrachloro-iodate (III), KICl4. This salt also sometimes is called potassium tetrachloroiodide, but this name is not really good. Iodine is not in oxidation state -1, but in oxidation state +3. The chlorine is in oxidation state -1. This salt can be regarded as a salt of the complex ion [ICl3.Cl]. This ion easily decomposes, giving off ICl3.
![]() In this experiment some free chlorine gas is formed. The amount is not large
enough to be really dangerous, but it is sufficient to make you feel very
uncomfortable if this experiment is not performed in a sufficiently ventilated
place. Standard kitchen exhaust is not really sufficient, it is best to perform
this experiment outside or in a fume hood.
In this experiment some free chlorine gas is formed. The amount is not large
enough to be really dangerous, but it is sufficient to make you feel very
uncomfortable if this experiment is not performed in a sufficiently ventilated
place. Standard kitchen exhaust is not really sufficient, it is best to perform
this experiment outside or in a fume hood.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium iodate
-
concentrated hydrochloric acid of good purity
-
acetone
-
sodium sulfite
-
dilute hydrogen peroxide
-
sulphuric acid
![]() Required
equipment:
Required
equipment:
-
test tube
-
petri dish
![]() Safety:
Safety:
-
Concentrated hydrochloric acid is corrosive and gives off highly irritating fumes, which should not be breathed.
-
 Concentrated sulphuric acid is extremely corrosive.
Concentrated sulphuric acid is extremely corrosive. -
Acetone is flammable.
![]() Disposal:
Disposal:
-
After the experiment, dilute with a lot of water, add a small amount of sodium sulfite (neutralization of iodine and chlorine) and let stand outside with air contact for a few hours (allowing most acetone to escape into the air), then carefully flush down the drain and rinse afterwards with a lot of water.
![]()
Preparation of the salt KICl4
![]() Take
a spatula full of solid potassium iodate and add 2 ml of concentrated
hydrochloric acid. This causes the liquid to foam and some gas is produced. This
gas is chlorine. So, it is best to perform this step outside or in a fume hood.
At least, there should be very good ventilation! The liquid becomes golden
yellow, that color is due to formation of the golden yellow ion ICl4.
The foaming can be quite vigorous, but it is not violent and no noticeable heat
is produced.
Take
a spatula full of solid potassium iodate and add 2 ml of concentrated
hydrochloric acid. This causes the liquid to foam and some gas is produced. This
gas is chlorine. So, it is best to perform this step outside or in a fume hood.
At least, there should be very good ventilation! The liquid becomes golden
yellow, that color is due to formation of the golden yellow ion ICl4.
The foaming can be quite vigorous, but it is not violent and no noticeable heat
is produced.
![]() After the foaming stops, heat the liquid in order to dissolve
the last amounts of solid. Do not boil vigorously, that will destroy most of the
ICl4,
and will drive off chlorine gas.
At this point you will have a small amount of clear golden yellow liquid. The
color is really beautiful.
After the foaming stops, heat the liquid in order to dissolve
the last amounts of solid. Do not boil vigorously, that will destroy most of the
ICl4,
and will drive off chlorine gas.
At this point you will have a small amount of clear golden yellow liquid. The
color is really beautiful.
![]() Put the warm test tube aside and allow it to stand for a few
hours. Do not cap the test tube, still small amounts of chlorine are released
slowly. After a few hours the solid potassium tetrachloro-iodate (III) has
settled and beautiful needle-shaped crystals are formed. The glass of the upper
part of the test tube also is covered by nice needle-like or even hair-like crystals.
Put the warm test tube aside and allow it to stand for a few
hours. Do not cap the test tube, still small amounts of chlorine are released
slowly. After a few hours the solid potassium tetrachloro-iodate (III) has
settled and beautiful needle-shaped crystals are formed. The glass of the upper
part of the test tube also is covered by nice needle-like or even hair-like crystals.
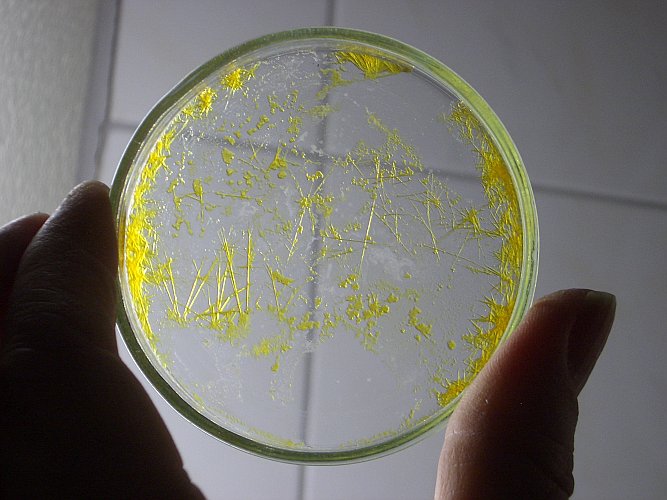
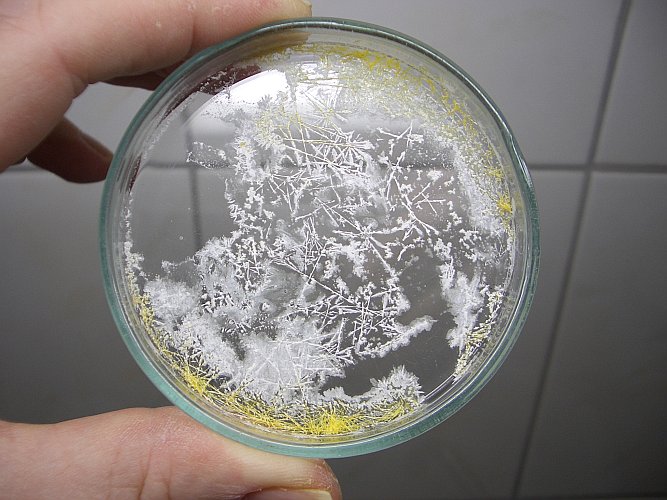
The following pictures show the crystals. For a more detailed picture, click on the small pictures below:
The left picture was made with light, going through the liquid and that nicely shows the glittering crystals. The middle picture was made with light from behind. The crystals look more yellow and that indeed is their real color.
![]() Decant the golden yellow liquid as much as possible. This allows the crystals to
be observed in more detail. The golden yellow liquid can be added to a small
amount of a solution of sodium sulfite in water and then this solution can be disposed of
by flushing it down the drain. The crystals should be kept. These crystals look
really neat. These are crystals of solid KICl4.
Decant the golden yellow liquid as much as possible. This allows the crystals to
be observed in more detail. The golden yellow liquid can be added to a small
amount of a solution of sodium sulfite in water and then this solution can be disposed of
by flushing it down the drain. The crystals should be kept. These crystals look
really neat. These are crystals of solid KICl4.

![]() The salt KICl4 is very reactive. This is shown by adding some acetone
to the still wet crystal mass. In the reaction, the crystal structure is broken
completely. It is quite remarkable that a crystalline salt structure is
completely destroyed by rinsing with acetone. Normally, rinsing with acetone is
a fairly good way to get rid of water, sticking to salt-crystals. When the
acetone is added, then at once, the solid breaks down and a white flocculent
precipitate is formed in a yellow liquid. The white solid can be observed on the
glass and it looks yellow in the acetone. The yellow color also changes from
golden yellow to brighter lemon yellow. The picture below shows the contents of
the test tube, immediately after some acetone is added to the crystal mass and
the contents was shaken. The precipitate settles very quickly after shaking.
The salt KICl4 is very reactive. This is shown by adding some acetone
to the still wet crystal mass. In the reaction, the crystal structure is broken
completely. It is quite remarkable that a crystalline salt structure is
completely destroyed by rinsing with acetone. Normally, rinsing with acetone is
a fairly good way to get rid of water, sticking to salt-crystals. When the
acetone is added, then at once, the solid breaks down and a white flocculent
precipitate is formed in a yellow liquid. The white solid can be observed on the
glass and it looks yellow in the acetone. The yellow color also changes from
golden yellow to brighter lemon yellow. The picture below shows the contents of
the test tube, immediately after some acetone is added to the crystal mass and
the contents was shaken. The precipitate settles very quickly after shaking.

After a certain induction time of a few tens of seconds, the liquid quite suddenly changes color. It also heats up noticeably at this point. It becomes warm, not hot. The following two pictures show the color of the liquid, approximately 60 seconds after adding the acetone and 90 seconds after adding the acetone. The color of the solid is hard to determine, because of the dark color of the liquid.


The precipitate is flocculent. When the liquid is shaken, then the precipitate quickly settles at the bottom (a matter of seconds), but it does not become really compact. The picture below shows the dark liquid and the precipitate, approximately 15 seconds after shaking.

![]() Add much
more water to the mix. When this is done, then the acetone dissolves in the
water and the precipitate also dissolves. The liquid becomes light brown and
(almost) clear.
Add much
more water to the mix. When this is done, then the acetone dissolves in the
water and the precipitate also dissolves. The liquid becomes light brown and
(almost) clear.
![]()
Drying and isolation of KICl4
In this second experiment with the salt, it is allowed to dry in contact with dry air. This experiment nicely shows that the salt is unstable, when allowed to stand in contact with air. The salt is not hygroscopic, but it looses ICl3, finally being turned into KCl.
Below follows a description of the experiment, where the salt is isolated and dried.
![]() Add a spatula full of KIO3 to concentrated HCl (30%) and heat, until all solid
has dissolved. Try to make the solution as concentrated as possible.
Add a spatula full of KIO3 to concentrated HCl (30%) and heat, until all solid
has dissolved. Try to make the solution as concentrated as possible.
![]() Pour the clear, still hot, and golden yellow liquid into a pre-heated petri dish
(heated under hot tap water and carefully dried, temperature is approximately 60
°C) and divide the liquid over the total surface of the petri dish, such that it
is covered by a very thin layer of the liquid. Keep the petri dish open, but
assure that no dust can come into the petri dish.
Pour the clear, still hot, and golden yellow liquid into a pre-heated petri dish
(heated under hot tap water and carefully dried, temperature is approximately 60
°C) and divide the liquid over the total surface of the petri dish, such that it
is covered by a very thin layer of the liquid. Keep the petri dish open, but
assure that no dust can come into the petri dish.
After a few tens of minutes, a nice set of needle-like crystals are formed on
the surface of the glass.
![]() Beware, a strong chlorine-like smell is produced in this step, the smell is
irritating, or even choking.
Beware, a strong chlorine-like smell is produced in this step, the smell is
irritating, or even choking.
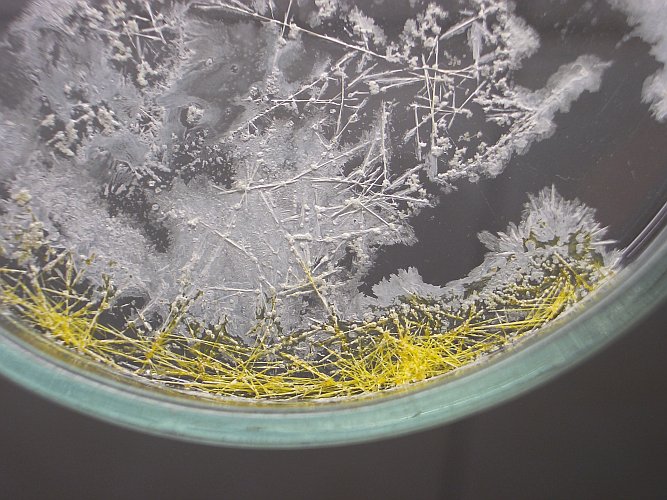
Below, four pictures are shown of the needle-like crystals. The left pictures show the entire petri dish and the right pictures show a detail of some nice crystals. Click on one of the pictures, for a larger and more detailed version. The bottom two pictures were made outside, with the blue sky as background.
These yellow crystals keep a strong chlorine-like smell and that indicates that they are decomposing.
![]() Allow the crystals to stay in contact with air for a
period of 10 hours at a temperature of 15
°C and at fairly low humidity (the weather was fairly cold and frosty at the
time the experiment was conducted). After this, the contents of the petri dish
has changed considerably. A large part of the crystals has become white, but
still there is quite some yellow material at the rims, where there was a thicker
layer of needle-like crystals (again click the pictures for more detail):
Allow the crystals to stay in contact with air for a
period of 10 hours at a temperature of 15
°C and at fairly low humidity (the weather was fairly cold and frosty at the
time the experiment was conducted). After this, the contents of the petri dish
has changed considerably. A large part of the crystals has become white, but
still there is quite some yellow material at the rims, where there was a thicker
layer of needle-like crystals (again click the pictures for more detail):
At this point, most of the yellow material was scraped out of the petri dish, assuring that as little as possible of the white material is scraped together with the yellow material. However, quite some yellow material was left in the petri dish as well, because it was too hard to separate this from the white material. The yellow material was collected on a piece of white paper. Total amount was approximately 100 mg. A picture of the material is shown below. The material is perfectly dry and crystalline, and has a strong, choking chlorine-like smell (it is, however, not exactly like chlorine, it resembles it). A crystal of this yellow material, rubbed between fingers, gives yellow/brown stains on the skin, much like stains of iodine.

After making this picture, this material was put in a tightly capped vial. The white material and the remaining yellow material was kept in the petri dish, with the petri dish still open.
![]() Keep the closed vial with yellow crystals for another 14 hours and also keep the
petri dish with the white material and the yellow remains for another 14 hours.
Keep the closed vial with yellow crystals for another 14 hours and also keep the
petri dish with the white material and the yellow remains for another 14 hours.
After this additional period of 14 hours, the contents of the petri dish has become completely white, the remaining yellow material now also has become white. The crystals in the petri dish also seem to be powdery. They have kept their shape, but they look powder-like. A detailed picture of the powder-like once-crystalline mass is shown here. It looks very neat. This picture was made with a fairly dark grey background.

So, all yellow material has decomposed to a white solid. The white solid is totally odorless and non-hygroscopic.
The contents of the vial still is yellow after these 14 hours. It remains yellow, also after weeks of storage, so in a tightly capped vial it can be stored indefinitely. The two pictures below show the contents of the vial after 14 hours:
The picture below shows another somewhat larger sample, after storing it in a vial for a few weeks:

![]() Scrape off the white contents of the petri dish. This gives a piece of white
non-crystalline, but perfectly dry powder. This powder was subjected to the
following tests:
Scrape off the white contents of the petri dish. This gives a piece of white
non-crystalline, but perfectly dry powder. This powder was subjected to the
following tests:
- Add some of the powder to concentrated H2SO4: This gives formation of a colorless gas, which fumes in contact with humid air and has a pungent smell. This gas most likely is HCl, this would mean that the compound contains quite some KCl.
- Dissolve some of the powder in water and add a small amount of dilute H2SO4. Next, add some 3% H2O2. After this, the liquid still is colorless. This means that hardly any (or not at all) iodide is present in the white powder. Even small amounts of iodide would be detected, due to formation of a yellow or yellow/brown liquid.
- Dissolve some of the powder in water and add a small amount of dilute H2SO4. Next, add a tiny amount of solid Na2SO3. The crystals of Na2SO3 dissolve and the liquid remains colorless. This indicates that no iodine is present in higher oxidation states than 1. E.g. addition of sulfite to excess acidified iodate solutions results in formation of brown/black iodine, but such a thing could not be observed here at all.
![]() A counter experiment was done with the yellow
crystals.
A counter experiment was done with the yellow
crystals.
- Dissolve some of the yellow solid in water and add a small amount of dilute H2SO4. This results in formation of a yellow/brown solution. Next, add a tiny amount of solid Na2SO3. The crystals of Na2SO3 dissolve and the liquid becomes turbid and dark brown. Solid iodine is formed. This is a clear indication that the yellow solid contains iodine in a oxidation state, larger than 1.
From all the tests with the white powder and the fact, that the only metal ion in solution is from potassium, one can safely conclude that the white powder is KCl, without any appreciable amount of iodine in it, in the form of iodide or iodate.
![]()
Discussion of results
![]() When potassium iodate is added to concentrated
hydrochloric acid, then it is capable of oxidizing the acid. This reaction is
fairly vigorous. The oxidation products are chlorine gas and the tetrachloro-iodate
(III) ion. The iodine goes from oxidation state +5 to oxidation state +3.
When potassium iodate is added to concentrated
hydrochloric acid, then it is capable of oxidizing the acid. This reaction is
fairly vigorous. The oxidation products are chlorine gas and the tetrachloro-iodate
(III) ion. The iodine goes from oxidation state +5 to oxidation state +3.
KIO3(s) + 6H+(aq) + 6Cl(aq) → K+(aq) + ICl4(aq) + Cl2(g) + 3H2O(l)
On cooling down, the solid potassium tetrachloro-iodate (III) crystallizes:
K+(aq) + ICl4(aq) → KICl4(s)
![]() When acetone is added to the solid, then it decomposes
again, probably as follows:
When acetone is added to the solid, then it decomposes
again, probably as follows:
KICl4(s) → KCl(s) + ICl3(dissolved)
This may explain the bright yellow color, ICl3 is lemon-yellow. This, however, is just my theory. It looks plausible, but another explanation may also be valid for the observations.
Whatever the precise properties of the yellow compound in the acetone, it does contain iodine. On standing, it reacts with the acetone, leaving iodine behind. The dark reddish/brown color is due to dissolved iodine in acetone.
![]() When the yellow KICl4(s) is
allowed to stand in contact with air, then it slowly decomposes, giving vapor of
ICl3, which in turn quickly decomposes to ICl and Cl2 (see
experiment with ICl and ICl3),
explaining why the smell is chlorine-like, but not exactly like chlorine. The
remaining solid is KCl.
When the yellow KICl4(s) is
allowed to stand in contact with air, then it slowly decomposes, giving vapor of
ICl3, which in turn quickly decomposes to ICl and Cl2 (see
experiment with ICl and ICl3),
explaining why the smell is chlorine-like, but not exactly like chlorine. The
remaining solid is KCl.
![]() When sulfite is added to an excess amount of a solution of
KICl4(s) in water,
then the liquid becomes dark brown and solid iodine is formed:
When sulfite is added to an excess amount of a solution of
KICl4(s) in water,
then the liquid becomes dark brown and solid iodine is formed:
2ICl4(aq) + 3SO32(aq) + 3H2O(l) → I2(s,aq) + 3SO42(aq) + 6H+(aq) + 8Cl(aq)
All other reaction products are colorless and cannot be observed.
![]() When a small amount of
KICl4(s) is dissolved
in water, then the liquid becomes light yellow/brown. The brown component of the
color most likely is due to hydrolysis and disproportionation at the low
concentration of such dilute solutions. The I2, formed in this
reaction, gives the brown color.
When a small amount of
KICl4(s) is dissolved
in water, then the liquid becomes light yellow/brown. The brown component of the
color most likely is due to hydrolysis and disproportionation at the low
concentration of such dilute solutions. The I2, formed in this
reaction, gives the brown color.
5ICl4(aq) + 9H2O(l) ↔ I2(aq) + 3IO3(aq) + 18H+(aq) + 20Cl(aq)
This reaction, however, certainly is not complete. Even with a fairly large quantity of the yellow solid, only a weak brown color can be observed. So, the compound is susceptible to hydrolysis, but not to such an extent, that it cannot be in aqueous solution at all.
![]()
Remark: The experiment can also be done with potassium periodate instead of potassium iodate. In that case the reaction with concentrated hydrochloric acid is as follows:
KIO4(s) + 8H+(aq) + 8Cl(aq) → K+(aq) + ICl4(aq) + 2Cl2(g) + 4H2O(l)
Remark
2: Cesium in its +1 oxidation state is even more suitable for making polyhalide
compounds than potassium in its +1 oxidation state. Cesium allows stabilization
of the

 ions
[Br2·Br]
and [BrCl·Cl],
which are not stable in aqueous solution, but which are perfectly stable in the
crystal lattice with the large Cs+ ion. The compound CsBr3
is totally stable in air, it has no smell of bromine. The stability of the
compound CsBrCl2 is comparable with the stability of KICl4.
Making of these compounds is remarkably easy. Putting finely powdered solid CsBr
in some excess liquid bromide and subsequent gentle heating to drive off excess
bromine is all, which is needed to make CsBr3. Making CsBrCl2
is as simple as leading slightly humid chlorine gas over finely powdered solid
CsBr, followed by very gentle heating in an atmosphere of chlorine gas. No
bromine is released, chlorine is absorbed instead and CsBrCl2 is
formed. It is remarkable to see that the colors of the salts are
much lighter than the colors of liquid bromine and bromine chloride. The orange
compound is the tribromide, the yellow compound is the dichlorobromide.
ions
[Br2·Br]
and [BrCl·Cl],
which are not stable in aqueous solution, but which are perfectly stable in the
crystal lattice with the large Cs+ ion. The compound CsBr3
is totally stable in air, it has no smell of bromine. The stability of the
compound CsBrCl2 is comparable with the stability of KICl4.
Making of these compounds is remarkably easy. Putting finely powdered solid CsBr
in some excess liquid bromide and subsequent gentle heating to drive off excess
bromine is all, which is needed to make CsBr3. Making CsBrCl2
is as simple as leading slightly humid chlorine gas over finely powdered solid
CsBr, followed by very gentle heating in an atmosphere of chlorine gas. No
bromine is released, chlorine is absorbed instead and CsBrCl2 is
formed. It is remarkable to see that the colors of the salts are
much lighter than the colors of liquid bromine and bromine chloride. The orange
compound is the tribromide, the yellow compound is the dichlorobromide.