




Mercury(II) chlorite : a
beautiful lab curiosity
The compound, prepared in this experiment, is mercury(II) chlorite. It has a beautiful orange/red color, but it is very toxic and also easily decomposes, more or less explosively. I had an unexpected quick decomposition of solid mercury(II) chlorite, which resulted in the formation of a cloud of mercury(II) chloride, which is very toxic.
![]()
![]() Required chemicals:
Required chemicals:
- mercury metal
- concentrated nitric acid (home made
acid of any concentration between 50% to 70% is
suitable)
- sodium hydroxide
- sodium chlorite
- concentrated hydrochloric acid
![]() Required equipment:
Required equipment:
- small beaker or erlenmeyer
- test tubes
- petri dish
- plastic spatula
![]() Safety:
Safety:
- Mercury metal is toxic and one must be very careful not to spill any metal on the floor.
- Nitric acid is very corrosive. Avoid contact with skin and eyes.
- Sodium hydroxide is corrosive. Avoid contact with skin and eyes.
- Sodium chlorite is a strong oxidizer. Mixes with reducing agents are quite sensitive and may ignite by friction.
- Concentrated hydrochloric acid is corrosive and gives off corrosive and choking fumes.
 The chemical, made in
this experiment, is very unstable and for this reason
the experiment should not be scaled up.
Unexpected violent decomposition may occur, which may
have dire consequences if this occurs with large
quantities.
The chemical, made in
this experiment, is very unstable and for this reason
the experiment should not be scaled up.
Unexpected violent decomposition may occur, which may
have dire consequences if this occurs with large
quantities.
![]() Disposal:
Disposal:
- The waste of this experiment may
not be flushed down the drain! Mercury waste is very
toxic and has severe long-term environmental
consequences for aquatic life. Bring the waste to a
proper municipal waste processing facility.
![]()
Preparation of a weakly acidic
solution of mercury nitrate
For this experiment, a small mercury
switch is used, which can be purchased on eBay. A lot of
100 pieces can be bought for 15 to 20 euros, including
the cost of shipping. The switches look like this:

These switches have a diameter of 5 or
6 mm and the length of the glass tube is appr. 1 cm.
From such a switch, the top can be taken off, using a
metal clamp, without shattering the entire glass tube.
After removing the top, the blob of mercury must be
transferred to a test tube.
Whatever method is used for getting the mercury out
of the switch, do this in a plastic tub, such that if
the switch shatters and the mercury is spilled, it
does not spill on the workbench or on the floor. A
drop of mercury easily breaks apart into a bazillion
of small droplets, which can be very hard to remove
and may get in cracks of the floor, workbench or wall.
The particular switch, which I used, had a globule of
172 mg of mercury. The actual amount in such switches
may vary, any amount between 150 mg and 250 mg is
possible.
![]() In another test
tube, approximately 1 ml of nitric acid must be heated
till it is close to boiling. The acid must be fairly
concentrated. The acid, used in this experiment, was
home made by means of distillation, and diluted with
water to get a concentration of 60% or so. Any
concentration between 50% and 70% by weight is suitable,
it is not very critical. Too low a concentration must be
avoided, because then part of the mercury may be
converted to mercury(I) and too high a concentrated may
lead to problems in getting the mercury dissolved
easily.
In another test
tube, approximately 1 ml of nitric acid must be heated
till it is close to boiling. The acid must be fairly
concentrated. The acid, used in this experiment, was
home made by means of distillation, and diluted with
water to get a concentration of 60% or so. Any
concentration between 50% and 70% by weight is suitable,
it is not very critical. Too low a concentration must be
avoided, because then part of the mercury may be
converted to mercury(I) and too high a concentrated may
lead to problems in getting the mercury dissolved
easily.
The blob of mercury must be added to the hot acid. The
mercury quickly dissolves in the acid, it takes one
minute or so to get it dissolved completely. The
resulting liquid is yellow, due to some dissolved
nitrogen dioxide. It is best to put a piece of paper
tissue in the open end of the test tube, while
dissolving the mercury in the acid. The dissolving of
the metal is accompanied by strong bubbling and this
brings an aerosol, containing mercury ions, into the
air. If a piece of paper tissue is put in the open end
of the test tube, then any gas (mainly air and nitrogen
dioxide) can easily escape, while droplets, containing
mercury, are absorbed by the paper tissue.
In the hot concentrated acid, all mercury metal is
oxidized to mercury(II).
![]() Once
all mercury has dissolved, dilute the liquid with appr.
10 ml of water.
Once
all mercury has dissolved, dilute the liquid with appr.
10 ml of water.
![]() The
solution with mercury(II) ions is too acidic for the
remaining part of the experiment. It must be converted
to a much less acidic solution. The easiest way to do
that is first adding an excess amount of a dilute
solution of sodium hydroxide. This will precipitate all
mercury as yellow mercury(II) oxide.
The
solution with mercury(II) ions is too acidic for the
remaining part of the experiment. It must be converted
to a much less acidic solution. The easiest way to do
that is first adding an excess amount of a dilute
solution of sodium hydroxide. This will precipitate all
mercury as yellow mercury(II) oxide.

The yellow
precipitate should be allowed to settle. This takes a
few hours. After that, decant as much of the colorless
solution as possible. This colorless solution only
contains trace amounts of mercury and can be flushed
down the drain (assure that no yellow precipitate is
decanted). Next, add another 25 to 30 ml of distilled
water and stir. Allow the yellow precipitate to settle
again and decant again. After this treatment, most
sodium hydroxide is removed, but having a little
sodium hydroxide remaining in solution is not a
problem.
![]() Next,
add 1 ml of concentrated nitric acid to 10 ml of water
and carefully add this dilute acid to the wet yellow
precipitate, drop after drop, until all yellow
precipitate just dissolves. After each drop, wait a few
tens of seconds to allow part of the precipitate to
dissolve. Keep adding drops of dilute acid, until the
liquid is only slightly opalescent. After that, put it
aside. After a few tens of minutes, the opalescence is
gone and one has a completely clear colorless solution.
This solution is weakly acidic, and that is exactly what
is needed for the further steps in this experiment.
Next,
add 1 ml of concentrated nitric acid to 10 ml of water
and carefully add this dilute acid to the wet yellow
precipitate, drop after drop, until all yellow
precipitate just dissolves. After each drop, wait a few
tens of seconds to allow part of the precipitate to
dissolve. Keep adding drops of dilute acid, until the
liquid is only slightly opalescent. After that, put it
aside. After a few tens of minutes, the opalescence is
gone and one has a completely clear colorless solution.
This solution is weakly acidic, and that is exactly what
is needed for the further steps in this experiment.
Preparation of mercury(II) chlorite
In the next step of the experiment, mercury(II) chlorite is prepared by adding a large excess of sodium chlorite to the colorless solution of mercury(II) nitrate.
![]() Prepare a
fairly concentrated solution of sodium chlorite by
dissolving 1 gram or so of solid sodium chlorite
in 10 ml of water (the commercial product usually
contains approximately 80% of sodium chlorite).
Prepare a
fairly concentrated solution of sodium chlorite by
dissolving 1 gram or so of solid sodium chlorite
in 10 ml of water (the commercial product usually
contains approximately 80% of sodium chlorite).
![]() Slowly, while stirring, add the solution
of sodium chlorite to
the weakly acidic solution of mercury nitrate.
When this is done, a beautiful bright orange/red
precipitate is formed. This bright orange/red
compound is mercury(II) chlorite, Hg(ClO2)2.
After formation of the precipitate, add some
additional water and stir to mix the liquid and
the precipitate well.
Slowly, while stirring, add the solution
of sodium chlorite to
the weakly acidic solution of mercury nitrate.
When this is done, a beautiful bright orange/red
precipitate is formed. This bright orange/red
compound is mercury(II) chlorite, Hg(ClO2)2.
After formation of the precipitate, add some
additional water and stir to mix the liquid and
the precipitate well.

![]() Allow
the orange compound to settle at the bottom. The liquid
above the orange solid is yellow. This is due to the
presence of chlorine dioxide, which is formed from the
excess amount of chlorite ions in the solution, together
with the small amount of acid in the solution of
mercury(II) nitrate.
Allow
the orange compound to settle at the bottom. The liquid
above the orange solid is yellow. This is due to the
presence of chlorine dioxide, which is formed from the
excess amount of chlorite ions in the solution, together
with the small amount of acid in the solution of
mercury(II) nitrate.

This yellow color is a good indication. It means that the solution really was somewhat acidic, but not too much (otherwise the liquid would have an intense yellow color). The weak acidity is important. If the solution is not acidic, then the precipitate will not be pure mercury(II) chlorite, but it will be a basic precipitate, containing chlorite, but also oxide and/or hydroxide. The color of such a basic precipitate is less brilliant, it is more brown/orange instead of red/orange.
![]() Once the orange precipitate has settled,
decant the yellow liquid and add 30 ml
of distilled water and stir. Let the
precipitate settle again. After this
second step, the liquid will be nearly
colorless, or maybe very pale yellow,
depending on how well the liquid was
decanted after the first step. Again
decant the (nearly) colorless liquid.
Once the orange precipitate has settled,
decant the yellow liquid and add 30 ml
of distilled water and stir. Let the
precipitate settle again. After this
second step, the liquid will be nearly
colorless, or maybe very pale yellow,
depending on how well the liquid was
decanted after the first step. Again
decant the (nearly) colorless liquid.
The liquids can be decanted into the
sink, they hardly contain any mercury.
Just be careful not to decant some of
the orange precipitate into the sink
with the liquid.
Isolation of mercury(II)
chlorite
Finally, the mercury chlorite can be
isolated. After decanting the (nearly) colorless liquid,
a small volume of liquid, containing the orange
precipitate, is left.
![]() Pour
the liquid with the precipitate into a petri dish and
allow this to dry on a warm (but not hot) place, free of
dust.
Pour
the liquid with the precipitate into a petri dish and
allow this to dry on a warm (but not hot) place, free of
dust.
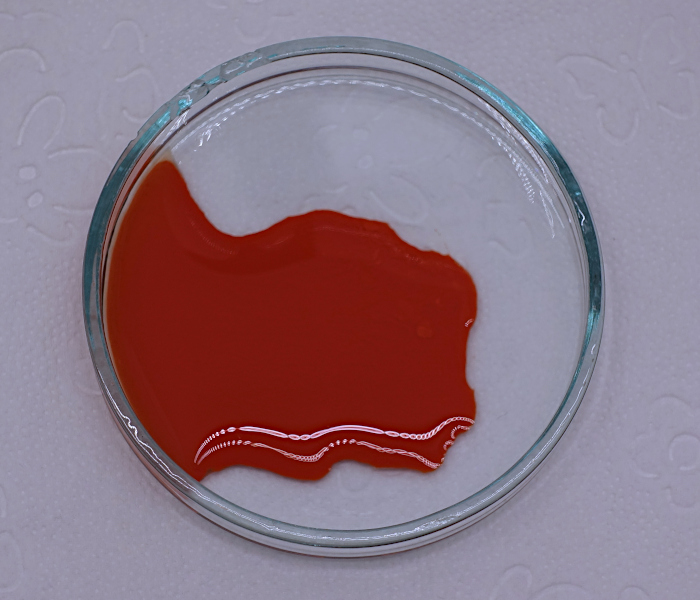
One day later, after drying, the solid looks as follows:
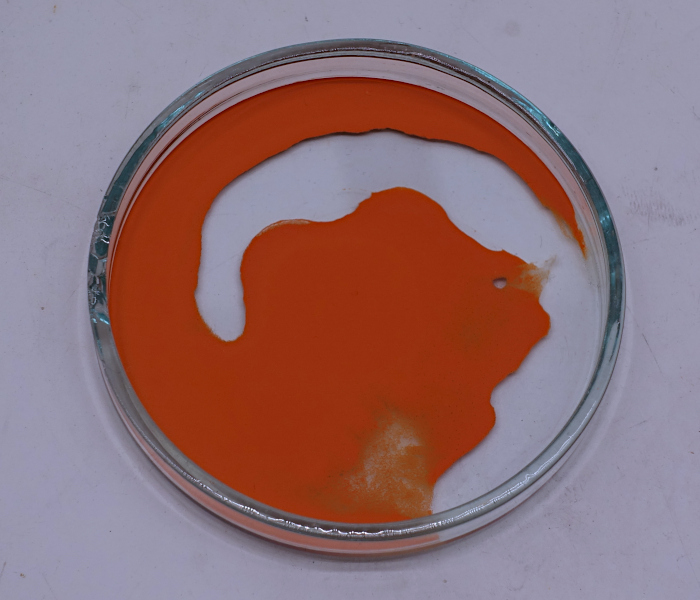
With a plastic spatula, the solid can
be scraped from the glass without any problems. This
scraping must be done carefully. If it is done too
wildly, then the material decomposes already while
scraping it from the glass. This is accompanied with
tiny high-pitched crackling noises. Do not collect all
of the solid on a single heap!
UNEXPECTED EXPLOSION!
The next step is collecting the solid
into a small vial. Only appr. 200 mg of solid is
obtained, so only a small vial is necessary. This is
where this experiment had an unexpected turn.
After scraping nearly all orange solid from the glass,
the solid was collected on one side of the petri dish
and a small funnel was used to collect the solid into
the small vial. When nearly all solid was in the vial
(appr. 3 mm layer of orange powder) it suddenly
exploded, giving a big cloud of white smoke and a high
pitched, not very loud bang. The explosion was not
violent, it was at the border of being a very fast
decomposition and being a true explosion.
Below follow pictures of the vial
after the explosion. There is no picture of the vial
with the orange powder, it exploded before a picture
could be made. The explosion occurred while the funnel
was still above the vial and was tapped a little to tap
off adhering orange powder into the vial.


After the white cloud was produced, I immediately kept my breath, opened the window, started a fan at full speed, closed the door and left the home lab for a while. The white smoke almost certainly is mercury(II) chloride, very toxic, not something you want to inhale!
The vial was not hot after the explosion (I had it in my hand, together with the funnel, while the explosion occurred and with the other hand I tapped the funnel). Apparently not much heat is produced in the decomposition reaction, but a big plume of white smoke was ejected from the vial. The funnel also was covered by a white layer of 'frost'.
After this failure, some concentrated hydrochloric acid was added to the frosty material in the vial. This results in immediate dissolving of the white solid and also of the orange specks. The solution is yellow, due to formation of chlorine dioxide.

In the petri dish a tiny amount of orange material was
left over. In the recorded
video it is demonstrated how easy it is to
decompose the orange material. The spatula, which
touches the orange powder is heated somewhat (close to
100 °C, most likely it cooled down to a little less
before it hit the orange powder) and as soon as it
touches the orange powder, it decomposes, giving white
smoke.
![]()
Discussion of results
In the first step, mercury metal is dissolved in hot fairly concentrated nitric acid. This results in the following reaction:Hg + 4 HNO3 → Hg2+ + 4 NO3 – + 2 H2O + 2 NO2
This reaction is carried out in a
large excess amount of acid. The mercury(II) ions are
precipitated with hydroxide ions and after that, the
resulting oxide is redissolved again with a small excess
amount of acid:
Hg2+
+ 2 OH– → HgO + H2O
The HgO precipitates as a yellow
solid. Probably it is in a hydrous form. With a little
acid, the yellow HgO is redissolved again to mercury(II)
ions in solution:
HgO + 2 H+ → Hg2+
+ H2O
At this point, there is a weakly
acidic solution of mercury(II) nitrate. This is treated
with a solution of sodium chlorite. The main reaction is
formation of the bright orange/red precipitate:
Hg2+ + 2 ClO2– → Hg(ClO2)2
The nitrate ions and sodium ions are
spectator ions and remain in solution. Because of the
acidity, this indeed is the main reaction, which occurs.
Without the acidity, a basic precipitate is formed,
which besides chlorite also contains oxide and/or
hydroxide.
There also is a side reaction.
Chlorite ion reacts with acid to form chlorous acid,
which is unstable and quickly decomposes. The net
reaction is:
5 ClO2– + 4 H+ → 4 ClO2 + Cl– + 2 H2O
This explains the
yellow color of the liquid above the red/orange
precipitate.
The compound Hg(ClO2)2
is unstable and easily decomposes. Slight heat
or other provocation causes it to decompose as
follows:
Hg(ClO2)2 → HgCl2 + 2 O2
With this
decomposition a lot of gas is produced and this
explains why so much smoke is expelled. The
mercury(II) chloride is expelled with the oxygen
gas as a white smoke and it settles on nearby
surfaces as a white frosty solid.