


Electrolysis with high voltages
This is a very interesting and very spectacular experiment, with very peculiar effects, but also with violent flashes and bangs. It is, however, also a very dangerous experiment, probably the most dangerous of all experiments in the chemistry section of this website. If one is not confident of himself/herself being capable of handling high voltage devices safely, please do not attempt to reproduce the results of this experiment.
In another page, the characteristics of an electrolysis cell are investigated. In that page the current as function of applied voltage is determined experimentally and a model is derived, based on the redox potential of the reactants, involved in the electrolysis. In this page, it is investigated, what happens when electrolysis is performed at much higher voltages, than the usual cell voltages. Here, many kilovolts are applied to the cell, instead of volts.
When very high voltages are applied to an electrolysis cell, then some really interesting phenomena can be observed, which vary strongly as function of the current limiting resistance, used in the wire between the power supply and the electrolysis cell.
In this experiment the following setup is used:
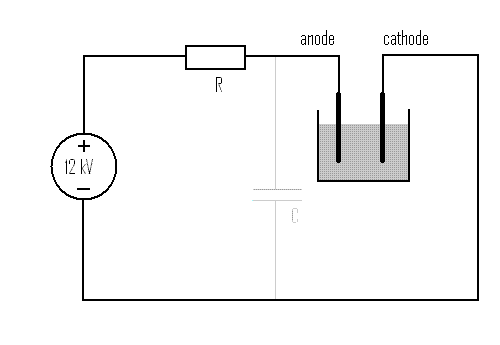
First, a set of experiment is performed with two different values of R, investigating what happens when the current is limited. Finally, an experiment is done, with the intent to have a very low value for R. However, for that experiment, R is not made really low, but instead, a high voltage capacitor C is used, which is fully charged, before the electrodes are placed in the liquid. This was done for safety reasons, not wanting to have disastrous effects to the power supply (or worse!) when the current can grow without limit. The results below show that this indeed was a very good idea!
In the experiments, where current was limited by R, there was no capacitor C.

 This
is a VERY dangerous experiment, it is the most dangerous of all experiments in
the chemistry section of this website. The voltages, used in this experiment are
very high and introduce a severe risk of possibly fatal electrical shock. When
the circuit is on, not a single part of the circuit may be touched, but one
should even stay away at least 10 cm from all active components and wires. At
the used voltage, sparks can go through the air and reach your body.
This
is a VERY dangerous experiment, it is the most dangerous of all experiments in
the chemistry section of this website. The voltages, used in this experiment are
very high and introduce a severe risk of possibly fatal electrical shock. When
the circuit is on, not a single part of the circuit may be touched, but one
should even stay away at least 10 cm from all active components and wires. At
the used voltage, sparks can go through the air and reach your body.
The capacitor, used in the experiments, is charged to 12 kV. The capacitor keeps this charge, a long time after the power source is shut down. A discharge of this capacitor through your body almost certainly is LETHAL!
![]()
![]() Required
chemicals:
Required
chemicals:
-
sodium chloride
-
optional: potassium iodide (add a small amount of potassium iodide, when you don't want formation of irritating elementary chlorine gas).
![]() Required
equipment:
Required
equipment:
-
petri dish
-
tub, with a diameter of 30 cm at least
-
 (*) resistor of
approximately 40 MΩ
(*) resistor of
approximately 40 MΩ -
 (*) resistor of
approximately 5 MΩ
(*) resistor of
approximately 5 MΩ -
 (*) capacitor of
approximately 0.04 μF
(*) capacitor of
approximately 0.04 μF -
high voltage power supply, 10 ... 15 kV
-
clamps, which absolutely surely provide sufficient isolation for voltages of 12 kV
![]() Safety:
Safety:
-
This is a VERY dangerous experiment. The voltages, used in this experiment are really high and can give a really nasty shock. Depending on the strength (effective internal resistance) of the high voltage power supply, a shock from this device may be anywhere between highly inconvenient to lethal.
-

 The capacitor,
used in this experiment, is charged to 12 kV. An accidental discharge of
this capacitor through your body is LETHAL.
The capacitor,
used in this experiment, is charged to 12 kV. An accidental discharge of
this capacitor through your body is LETHAL. -
During the experiment, small amounts of ozone are produced, if the components are not properly "rounded", due to corona discharges in air. If the components have no sharp edges, then the amount of corona discharge is not that large. Just to be on the safe side, it is best to perform the experiment in a well ventilated room.
-
When sodium chloride is used, then chlorine gas is formed at the anode. Chlorine is very poisonous and has a highly irritating smell. The amount, formed in this experiment only is very small, but it is best to have good ventilation anyway. When potassium iodide is added, then iodine is formed, but this remains in solution as a yellow/brown compound and does not give rise to formation of bad fumes or gasses.
-
The bangs, produced in the later part of this experiment, are very loud. When the capacitor is discharged accidentally at once, then material (e.g. water droplets, salt crystals) is sprayed around very violently. Eye protection is a must.
![]() Disposal:
Disposal:
-
The solution can be flushed down the drain without problem.
(*) ADDITIONAL SAFETY REMARKS
![]()
![]() The resistors and
capacitor must be constructed in a special way. It is absolutely necessary,
to use high voltage components. The materials from the standard electronics
parts shops are not suitable for these experiments and will certainly lead to
very bad accidents. Resistors will arc over and capacitors may explode. In this
experiment, for the approximate 40 MΩ resistor, a series connection of ten 3.9
MΩ resistors was used, where each 3.9 MΩ resistor was a 2 W device, capable of
withstanding 2 kV. The 5 MΩ resistor was made from 15 resistors of 330 kΩ, also
2 W pieces, capable of withstanding 1.5 kV. The capacitor was made by a series
connection of 8 polypropylene film capacitors of 0.33 μF / 2
kV, which can withstand immediate discharge with high current. Below follows a picture of how the single high voltage capacitor was
constructed from 8 smaller high current capacitors. A pen is placed besides the
string of capacitors, to give an impression of the size of the components. The
strings of resistors have similar size.
The resistors and
capacitor must be constructed in a special way. It is absolutely necessary,
to use high voltage components. The materials from the standard electronics
parts shops are not suitable for these experiments and will certainly lead to
very bad accidents. Resistors will arc over and capacitors may explode. In this
experiment, for the approximate 40 MΩ resistor, a series connection of ten 3.9
MΩ resistors was used, where each 3.9 MΩ resistor was a 2 W device, capable of
withstanding 2 kV. The 5 MΩ resistor was made from 15 resistors of 330 kΩ, also
2 W pieces, capable of withstanding 1.5 kV. The capacitor was made by a series
connection of 8 polypropylene film capacitors of 0.33 μF / 2
kV, which can withstand immediate discharge with high current. Below follows a picture of how the single high voltage capacitor was
constructed from 8 smaller high current capacitors. A pen is placed besides the
string of capacitors, to give an impression of the size of the components. The
strings of resistors have similar size.

When this string of capacitors is charged to 10 kV ... 15 kV, then a discharge through your body will lead to very serious injury and there is a great chance that the discharge will lead to immediate death! If one wants to keep such a string of capacitors for other experiments as well, then it is a wise thing to put so-called bleeder resistors over each separate capacitor. For these capacitors, it is best to place a series connection of four ordinary ¼ Watt carbon film 10 MΩ resistors between the legs of each capacitor. Such a string of resistors assures that the capacitors will be discharged to safe levels within a few tens of seconds after power down, while at the same time not impairing the effectiveness of the capacitors too much. In the experiments, described at this page, these bleeder resistors were not present. The string of capacitors was only used for this experiment, and after this, it was soldered into separate parts again.
![]()
Electrolysis with a plasma anode
In this experiment, the cathode is immersed in the liquid. The cathode is a simple metal wire, it does not matter what metal this is. The anode is a plasma beam, which is built up between a wire, carrying the 12 kV potential and the liquid, in which the cathode is immersed. The liquid is a highly concentrated solution of sodium chloride. Between the wire, moved closely to the liquid, and the power supply, there is a resistor of 40 MΩ.
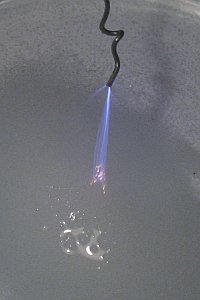
In the first experiment, the wire is moved towards the liquid (which in fact is table salt, totally soaked with water). When the distance is approximately 15 mm, then a wind is produced, which even can be felt easily, there also is a lot of hissing noise, and a blue/violet stream of plasma can be observed, starting at the wire, moving towards the liquid. When the wire is moved somewhat closer, then the blue/violet streams of plasma touch the liquid/salt mix. At places where they touch, there is a strong orange color. The surface of the liquid/salt mix is flickering and sparkling, wherever the gently moving and oscillating streams of plasma are touching it. This is shown in the picture below. The left wire is the cathode, the right wire, with the streams of plasma emerging from it, is the anode. In fact, the streams of plasma themselves are the anode. They are in contact with the liquid, while the wire is not in contact with the liquid. When this is allowed to continue for a while, then there is a strong smell of chlorine, but the smell never becomes so strong that it becomes really suffocating. This can be explained, because the current of this whole setup is at most approximately 300 μA.
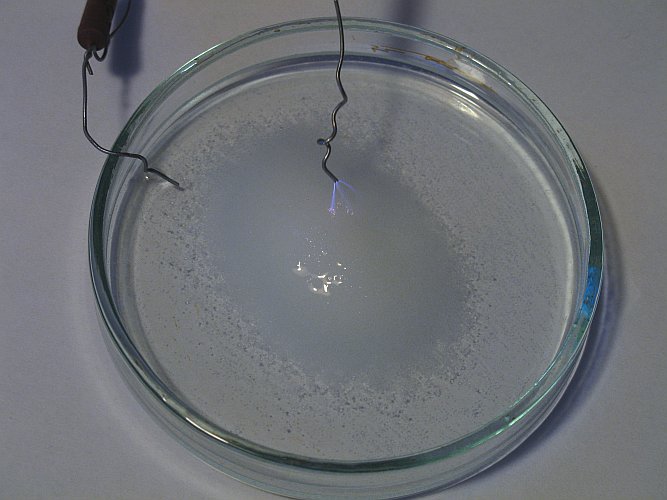
Below, a close-up is shown of the plasma, emerging from the anode-wire and the orange specks of light, where the streams of plasma touch the salt/water mix.

When the anode wire is moved closer to the liquid, then the plasma streams become more and more bundled, until a small, but well focused single stream of plasma is touching the liquid.
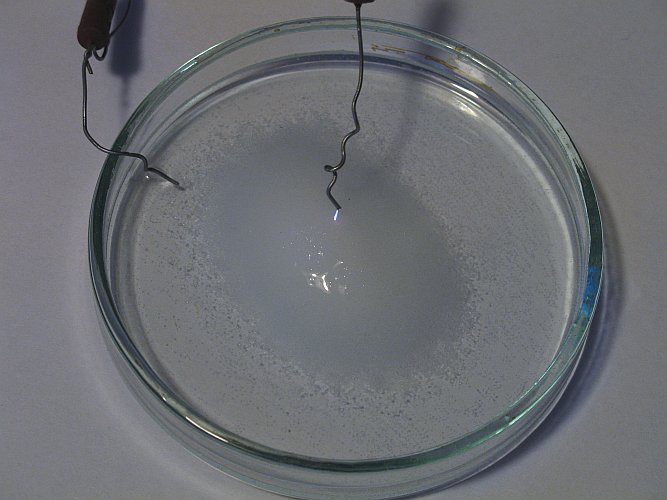
Effect of lower series resistance
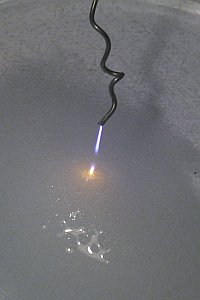
In the following experiment, the resistor of 40 MΩ is replaced by a resistor of 5 MΩ. Now a current of almost 2.5 mA can flow through the liquid and the plasma. Again, the result is similar to the effect above, but the effect is much more spectacular. There also is a much stronger smell of chlorine.
The following picture shows the result when the wire has a distance of approximately 15 mm from the salt/water mix. Again, at the place, where the plasma touches the liquid, there is a nice sparkling and flickering of orange specks. There also is a very loud hissing (at times even whining) noise. Near the liquid, there is a strong and irritating smell of chlorine, so this electrolysis definitely is capable of releasing elemental chlorine from an aqueous solution of a chloride.
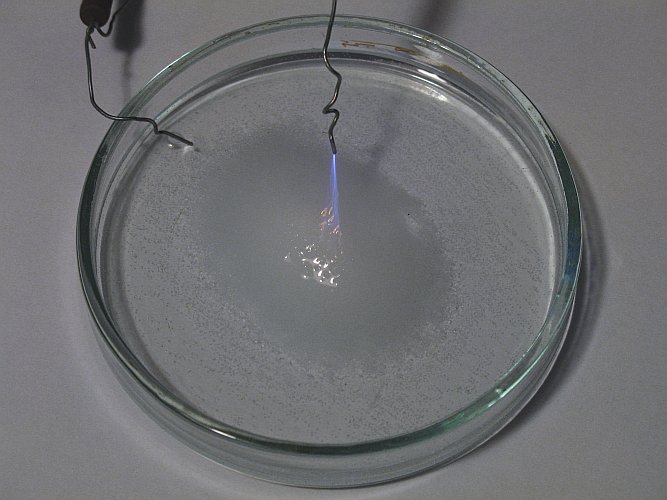
The effect becomes more spectacular, when the wire is slowly moved towards the liquid. The beam is focusing more and more and when the distance is approximately 12 mm, then a single bright and sharp beam of plasma is produced. At this point, the smell of chlorine seems even stronger. In the three pictures below, the wire was moved slowly towards the liquid and one can nicely see the plasma focus and become brighter. Near the liquid, the plasma becomes bright orange. Is this due to intense heat, or is this due to activation of sodium atoms, resulting in the well known orange/yellow color of sodium spectral lines? This was not investigated further, due to the high danger involved.


 A
really peculiar effect is obtained, when the distance is decreased even more.
The beam of plasma then seems to stop halfway between the wire and the liquid,
while at the liquid an orange beam appears. Sometimes the beam at the liquid is
very short and looks like a bright orange point. This effect is shown in the
picture at the left.
A
really peculiar effect is obtained, when the distance is decreased even more.
The beam of plasma then seems to stop halfway between the wire and the liquid,
while at the liquid an orange beam appears. Sometimes the beam at the liquid is
very short and looks like a bright orange point. This effect is shown in the
picture at the left.
The orange beam is not stationary, its length is changing randomly. It has a certain length for a few seconds, and then suddenly its length changes. Sometimes it touches the blue/violet beam. Sometimes it just is a bright spot at the liquid below, sometimes it even is gone. I have no explanation for this really peculiar effect. What is it?
This last set of experiments also was repeated by adding some potassium iodide to the table salt/water mix. If that is done, then the result is very similar. Again, the beam of plasma is split up when the wire is moved close to the liquid, and at higher distances, nice orange sparkling can be seen at the surface of the liquid. The only difference is that everywhere, where the plasma touches the liquid, it becomes yellow/brown at that point and no smell of chlorine can be observed anymore. The brown spots and streaks in the liquid make the experiment less attractive, but it is interesting to see that iodide also is oxidized, and no purple vapors of iodine can be observed. There is, however, unmistakenly, a smell of iodine.
Another interesting observation is that gas is produced everywhere in the petri dish very slowly. It seems as if the cathode reaction (formation of hydrogen from water) is not localized to the cathode wire, but everywhere at the bottom of the petri dish, there is very slow (but clearly noticeable) production of gas. When you look at the top part of the four pictures above, then little darker dots can be seen. These are not crystals of salt, but these are little gas bubbles, sticking to the bottom of the petri dish.
Effect of removing the series resistor
Finally, an experiment was done without limiting resistor. In order to protect the power supply and for safety, the series resistor was kept in place, but a capacitor was used, which was connected to both the cathode and anode wires. Again, the experiment was done with the same petri dish, with salt/water mix.
The result of this experiment was stunning and really shocking (in fact, a small accident happened). When the anode wire is moved towards the liquid slowly, then the result resembles that of the previous experiment. A fuzzy plasma is produced and nice orange sparkling can be observed at the surface of the liquid. At the point, where the plasma beam is going to focus to a narrow line, suddenly a very loud bang is produced, with a big and very bright spark from the center of the petri dish to the cathode. Salt and water is ejected from the petri dish with such high speed that it really hurts, where it hits the skin! Fortunately, the petri dish was not broken, otherwise glass would have been shattered around. The capacitor was discharged at once.
This experiment was not repeated anymore under these conditions. A new experiment was performed, however, with a much larger amount of tap water, and with no additional sodium chloride or potassium iodide added. In this way, a large path between anode and cathode can be assured (approximately 20 cm), and due to the low concentration of ionic compounds, the conductivity of the water is much lower.
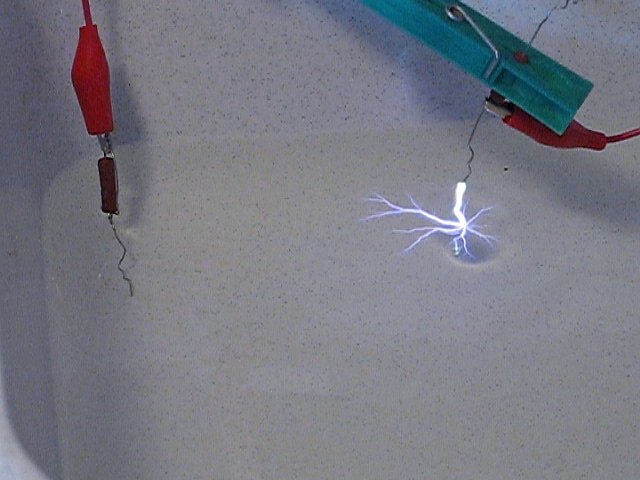
A tub was filled with a few cm of tap water, the cathode was immersed in the tap water, and the anode was slowly moved to the surface of the water, while the capacitor was fully charged (0.04 μF, 12 kV). This results in formation of a fuzzy plasma, moving from the anode towards the water. One can clearly see the effect of an ionic wind, which pushes the water downwards. No little orange sparkling could be observed now. The following picture shows the result of this. One can nicely see that the water is moving, due to the ionic wind.

When the anode wire is moved towards the water slowly, then suddenly again there is a really loud bang, and a big crackling spark is produced, which crawls over the surface of the water in all directions. This really is spectacular and also quite scary. The following picture shows the big spark and the sparks, going over the water surface. The spark from the wire to the water surface has a length of approximately 12 mm, the spark, going over the water surface has a length of approximately 3 cm, but there also were instances, where its size was almost 7 cm.
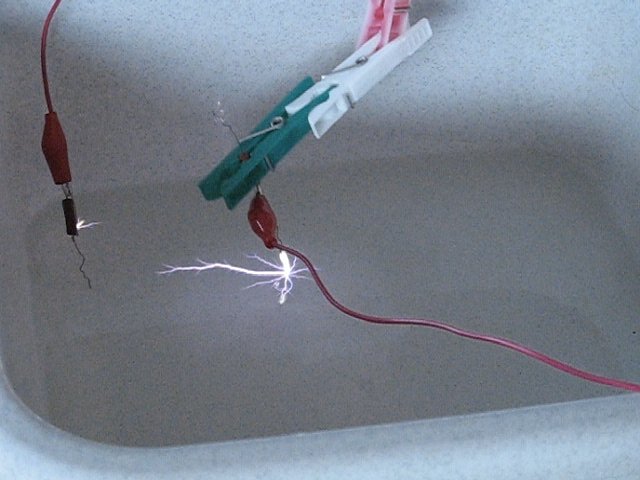
The following picture shows a much larger and much more powerful spark. This was produced, by moving the anode wire towards the liquid very quickly, assuring that almost no stored energy of the capacitor is used up by producing the ionic wind, as shown in the previous pictures. This spark produced a very loud bang. One can also see that a spark is formed at the cathode, along the surface of the water. When the cathode spark and anode spark meet, then effectively a short circuit is produced and the capacitor then discharges at once with a very loud bang and water is sprayed around violently. That was what happened in the experiment with the petri dish.
There are two little AVI movies of the creation of such big sparks. These sparks only exist for a short time and when they are created there is a loud bang. Click on the links below for the AVI movies. Both files have a size of approximately 600 kByte. Download speed is limited to 50 Kbyte per second:
![]()
Explanation of results
Chemically speaking, this reaction is quite interesting, but the precise compounds, formed at the plasma anode cannot be predicted easily.
At the wire, the following reaction occurs:
A – e → A+
Here, A is any molecule/atom of the air. Due to the extremely high electric field strength, atoms and molecules are broken apart. Electrons are ripped off and these are absorbed by the anode. The positively charged ions A+ are repelled from the anode. This repelling of these ions is so strong, that this effect can be observed very well. The water surface is pushed downwards (as if someone is blowing at the water surface with a little straw, a few cm above the surface). One can also feel the ionic wind (be VERY careful with that, only do this, when there is a big resistor of a few tens of MΩ in series with the power supply, and NO capacitor is connected).
At the cathode, the following reaction occurs:
2H2O + 2e → 2OH– + H2
The ions A+ move towards the surface of the liquid, causing an ionic wind (which can actually be felt from a distance of more than 10 cm), and at the surface they react with the water, and with the chloride or iodide in the water. The orange sparkling may be due to tremendous heat at the spots, where the ions A+ react with the chloride in the water, but more research is needed to confirm (or disprove) this. The following reactions undoubtedly will occur:
2A+ + 2Cl– → 2A + Cl2 (when iodide is present, replace Cl by I)
4A+ + 2H2O → 4A + 4H+ + O2
Almost certainly, there will be many side reactions. It is known that when A is an oxygen molecule, then it may react with other ions A, or with molecules in the air, before it even reaches the water surface. The result of these reactions is the formation of ozone, and possibly also of nitrogen oxides.
The plasma beam is a very good conductor of electricity. Due to the breakdown of air-molecules A into ions A+ and electrons, a current can flow easily. The electrons are moving towards the anode wire and absorbed by it, the positive ions move away from the wire.