


Extreme reactivity of
chlorosulfonic acid
Chlorosulfonic acid is a chemical,
which is used in industry on a large scale. In the
chemical lab it is much less common and this is for a
good reason. The compound is extremely corrosive and
very reactive with almost every other chemical,
including water and many common solvents and salts. In
this experiment, this extreme reactivity is demonstrated
in a beautiful way.
 Chlorosulfonic acid is extremely
corrosive. With water it reacts explosively. It eats
through skin in a fraction of a second. There will be NO
time to rinse away the acid or wipe off the acid! Avoid
contact with the human body! If you are not confident in
being capable of handling this chemical safely, then do
not perform this experiment.
Chlorosulfonic acid is extremely
corrosive. With water it reacts explosively. It eats
through skin in a fraction of a second. There will be NO
time to rinse away the acid or wipe off the acid! Avoid
contact with the human body! If you are not confident in
being capable of handling this chemical safely, then do
not perform this experiment.
![]()
![]() Required chemicals:
Required chemicals:
- chlorosulfonic acid
- potassium permanganate
- potassium dichromate
- sodium sulfite (metabisulfite or thiosulfate also is suitable) for cleaning up
- dilute sulfuric acid for cleaning up
![]() Required equipment:
Required equipment:
- big flask
- glass
pipette and balloon for conveniently transferring
corrosive liquids

![]() Safety:
Safety:
- Chlorosulfonic acid is extremely corrosive (see above).
- Potassium permanganate is a strong oxidizer, but in this experiment it is not used in a way, which introduces risk of accidental exothermic oxidizing reactions.
- Potassium dichromate is a known
carcinogen on inhalation. It also may act as a strong
sensitizer in some people. The fumes, produced in the
reaction with potassium dichromate are carcinogenic,
avoid exposure to these.
![]() Disposal:
Disposal:
- The waste can be reduced with a
solution of sodium sulfite or a solution of sodium
thiosulfate and can be flushed down the drain with a
lot of water.
![]()
Production of a mushroom
cloud
In this experiment, potassium permanganate (KMnO4) is mixed with chlorosulfonic acid (HSO3Cl). When these chemicals are mixed, then a violent reaction starts. Here it is described how this reaction can be performed safely on a small scale.
![]() Take a small spatula of
potassium permanganate and put this in the dry flask.
Assure that the solid is on a small pile. A round bottom
flask is most convenient, because that makes collecting
all solid at a single spot easy, just by swirling the
flask a little bit.
Take a small spatula of
potassium permanganate and put this in the dry flask.
Assure that the solid is on a small pile. A round bottom
flask is most convenient, because that makes collecting
all solid at a single spot easy, just by swirling the
flask a little bit.
![]() Put the flask in a clamp, such
that its contents can be observed well.
Put the flask in a clamp, such
that its contents can be observed well.
![]()
![]() Using a
glass pipette and a balloon, take 0.1 to 0.2 ml of
chlorosulfonic acid from its bottle. Do not pour the
acid and do not add more acid than the mentioned amount!
Pouring the acid introduces a high risk of accidents and
spills. Using larger amounts of acid makes
cleaning up the waste very troublesome, due to its
extremely violent reaction with water!
Using a
glass pipette and a balloon, take 0.1 to 0.2 ml of
chlorosulfonic acid from its bottle. Do not pour the
acid and do not add more acid than the mentioned amount!
Pouring the acid introduces a high risk of accidents and
spills. Using larger amounts of acid makes
cleaning up the waste very troublesome, due to its
extremely violent reaction with water!
![]()
![]() Drip the
liquid on the solid potassium permanganate.
Drip the
liquid on the solid potassium permanganate.
When the liquid touches the solid, a
brown/green fume is produced. It is not entirely clear,
whether this is a gas, or smoke. Most likely, this is a
gas, which also has white fumes of the acid mixed with
it. For a short time, more of the dense brown/green
gas/fume is produced, and then suddenly, there is a
small explosion, in which brown smoke is produced. This
brown smoke forms a mushroom cloud, which quickly moves
upwards. The pictures below show what happens.



The brown smoke later moves through the flask and thicker flakes of the solid fall back towards the bottom. Below follow a few links of high quality movies. One at real speed, one at half speed and one at 20% of real speed. Especially the latter nicely shows the formation of the mush room cloud and the formation of flakes, which fall back to the bottom:
Video
of reaction at 100% of real speed
Video of reaction at 50% of real speed
Video of reaction at 20% of real speed
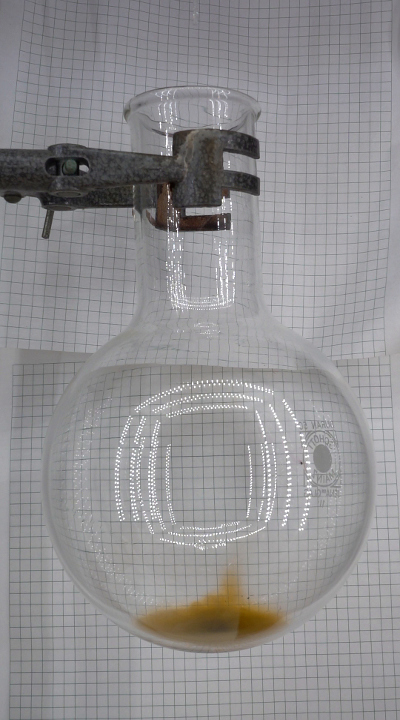
For cleaning up, prepare a solution of a few hundreds of mg of sodium sulfite in appr. 20 ml of water and add this to the flask. Swirl in order to get rid of all brown stains and let stand for an hour or so, covered with a petri dish, to allow all fumes to settle and dissolve in the water. The stains can be removed very easily with such a solution. After that, the flask can be cleaned further by rinsing with a lot of water. The waste can be flushed down the drain without problems for the environment.
![]()
Orange clouds in a flask.
![]() Take a spatula of potassium
dichromate and put this in the dry flask. A few hundreds
of mg of crystals is sufficient. As in the previous
experiment, assure that the solid is on a small pile,
covering a small area.
Take a spatula of potassium
dichromate and put this in the dry flask. A few hundreds
of mg of crystals is sufficient. As in the previous
experiment, assure that the solid is on a small pile,
covering a small area.
![]() Put the flask in a clamp, such
that its contents can be observed well.
Put the flask in a clamp, such
that its contents can be observed well.
![]()
![]() Using a
glass pipette and a balloon, take 0.2 to 0.3 ml of
chlorosulfonic acid from its bottle. Do not pour the
acid and do not add more acid than the mentioned amount!
Pouring the acid introduces a high risk of accidents and
spills. Using larger amounts of acid makes
cleaning up the waste very troublesome, due to its
extremely violent reaction with water!
Using a
glass pipette and a balloon, take 0.2 to 0.3 ml of
chlorosulfonic acid from its bottle. Do not pour the
acid and do not add more acid than the mentioned amount!
Pouring the acid introduces a high risk of accidents and
spills. Using larger amounts of acid makes
cleaning up the waste very troublesome, due to its
extremely violent reaction with water!
![]()
![]() Drip the
liquid on the solid potassium dichromate.
Drip the
liquid on the solid potassium dichromate.
In this
experiment, bright orange/red fumes are produced. These
fumes consist of a complicated mix of chromyl chloride,
fumes from the chlorosulfonic acid and the remarkable
compound chromyl chlorosulfonate, dissolved in
chlorosulfonic acid. All of these chemicals together
appear as an orange/red gas/fume.




The orange
fumes in this reaction are much more uniform than the
smoke in the reaction with potassium permanganate. The
fume slowly fills the flask. There also is formed more
orange gas during a few minutes. After the initial
violent reaction, the liquid at the bottom keeps on
releasing orange gas for several minutes. After 10
minutes, the flask is filled with a dense orange gas,
and the fumes have mostly settled at the glass.

These fumes are
quite dense and can be poured out of this flask. The
picture shows that inside the flask there is a gas and
not a fume and that the constituents of the fume (liquid
and/or solid matter) have settled in the lower part of
the flask on the glass wall. When the gas mix from the
flask is poured out in air, then the orange gas can be
observed, but there also is formation of new fumes. That
is most likely, due to presence of HCl in the gas mix
and due to hydrolysis of the orange CrO2Cl2-vapor
in the flask in contact with water vapor in the air.
Below follow a
few videos:
Video
of reaction at 100% of real speed
Video of reaction at 20% of real speed
Video
of pouring the vapor from the flask
For cleaning up, prepare a solution of half a gram or so of sodium sulfite in appr. 50 ml of dilute sulfuric acid (5% acid is OK) and add this to the flask. Swirl in order to dissolve all of the orange/red solid, sticking to the glass, and let stand for an hour or so, covered with a petri dish, to allow all fumes and vapor to settle and dissolve in the dilute acid. The solution in the flask will turn green. After that, the flask can be cleaned further by rinsing with a lot of water. If sufficient sodium sulfite is used (the liquid must be purely green), then the waste can be flushed down the drain without problems for the environment. Trivalent chromium is not particularly toxic and the small amount, used in this experiment, is no problem at all.
![]()
Discussion of results
Chlorosulfonic acid reacts with many acids, having OH-groups, by replacing the OH-group by a Cl-atom, itself being converted to sulfuric acid. An example is the reaction with HNO3 (which has structure O2N-OH).O2NOH + HOSO2Cl → O2NCl + HOSO2OH
With salts of oxoacids, however, a
similar reaction occurs, with formation of the metal
bisulfate. With potassium permanganate this leads to the
following reaction, written in a somewhat unconventional
form, which better shows the structure of the reactants
and products at the moment of reaction (one of the
resonance extremes of permanganate ion is O3MnO–):
O3MnOK + HOSO2Cl → O3MnCl + HOSO2OK
The volatile compound permanganyl
chloride, O3MnCl,
usually written as MnO3Cl, is very
unstable and it easily explosively decomposes to MnO2,
O2 and Cl2. At the start, fumes
and vapor of permanganyl chloride are formed, but
soon, the formed permanganyl chloride decomposes
nearly explosively, releasing a lot of gas and dark
brown solid
MnO2, which appears as a brown mushroom
cloud.
With potassium dichromate the reaction
is more complicated. With hexavalent chromium, a similar
reaction can occur as with permanganate, but
chlorosulfonic acid also can form chlorosulfonates with
hexavalent chromium. The first reaction is:
KO-CrO2-O-CrO2-OK + HOSO2Cl → KO-CrO2-O-CrO2-Cl + HOSO2OK
This compound, however, is
not stable, and immediately breaks down into different
compounds and through different intermediate steps, the
following compounds are formed:
- CrO2Cl2 (chromyl chloride, deep orange/red vapor)
- KOCrO2Cl (potassium chlorochromate, an orange/red solid)
- CrO2(OSO2Cl)2 (chromyl chlorosulfonate, dissolved in excess HSO3Cl)
- there might also be mixed
chloride chlorosulfonate chromyl
All of these compounds appear in the red/orange gas/fumes. Finally, when all liquid and solid matter has settled, only CrO2Cl2 remains present in the gas phase.