


Dehydrating sugar by sulphuric acid
The experiment, described in this page, is a well-known experiment, but also a really spectacular and appealing experiment. For that reason, it is added to the set of experiments, and this is something which really is worth doing, and it only requires very common chemicals, being plain sugar and concentrated sulphuric acid (drain cleaner grade is sufficient). Sugar and sulphuric acid simply are mixed and then the reaction is watched from a safe distance.

The experiment MUST be performed outside. It produces lots of smoke, and hot acid, loaded with charred sugar is splashing around. So, do this on a useless piece of concrete or something else, which may be spoilt. The bottle, in which the experiment is performed also will be useless after the experiment, so don't use your nice laboratory glassware for this.
![]()
![]() Required
chemicals:
Required
chemicals:
-
sugar
-
concentrated sulphuric acid
![]() Required
equipment:
Required
equipment:
- bottle, which may be thrown away, volume approximately 100 ml
- concrete tile, or other base, which also may be thrown away after the experiment
![]() Safety:
Safety:
- This reaction is violent and a lot of smoke is produced. Also acid and charred sugar are splashed around. This reaction MUST be performed outside. Beware, the smoke has a strong smell, it has a smell, which is a mix of the smell of sulphur dioxide and caramel. It is rather pungent.
![]() Disposal:
Disposal:
- Let the charred material in its bottle (or what remains of that) cool down, wrap in a plastic bag, add some powdered chalk or powdered sodium bicarbonate and throw in the household waste bin. There is no need to treat this waste as chemical waste. The chalk or sodium bicarbonate neutralizes the sulphuric acid, which still is in the charred solid matter.
![]()
Setting up for the experiment
![]() Take
approximately 75 grams of plain sugar. If the sugar consists of large crystals,
then crush them, but the sugar should not be powdered.
Put the sugar in the bottle.
Take
approximately 75 grams of plain sugar. If the sugar consists of large crystals,
then crush them, but the sugar should not be powdered.
Put the sugar in the bottle.
![]() Take
some sulphuric acid of at least 90% concentration. The acid should not be
ice-cold, so if it was stored in a cold place, then make it luke-warm, by
putting the bottle of acid in some warm water for half an hour.
Take
some sulphuric acid of at least 90% concentration. The acid should not be
ice-cold, so if it was stored in a cold place, then make it luke-warm, by
putting the bottle of acid in some warm water for half an hour.
![]() Put the
bottle with sugar, OUTSIDE, on a concrete tile or old piece of wood, which may
be thrown away after the experiment.
Put the
bottle with sugar, OUTSIDE, on a concrete tile or old piece of wood, which may
be thrown away after the experiment.
![]() Pour some of the acid in the sugar. Add acid, such that the
sugar becomes totally soaked with the acid, but the amount of acid should not be
so large, that a thick layer of acid is standing above the soaked sugar.
Pour some of the acid in the sugar. Add acid, such that the
sugar becomes totally soaked with the acid, but the amount of acid should not be
so large, that a thick layer of acid is standing above the soaked sugar.
![]()
Watching the experiment
After the acid has been added, step back. It takes some time before the reaction sets off. First, the sugar/acid mix darkens, but at a certain point, it starts bubbling and its volume strongly expands. This is due to formation of gas and due to rise of temperature. The total time of the reaction is a few minutes. Below, a set of pictures is given which shows different stages in the reaction. The first picture shows the sugar, before adding the acid.







Two videos are available of this experiment. The first
video shows the beginning, the adding of the acid to the sugar and the start of
the reaction. The second video shows the main part of the reaction, with the
black matter puffing out smoke and gases at every pore it has. There is a lot of
noise in the video, this is because it was made outside, with wind, traffic, and
my daughter watching the reaction
![]() .
Beware, the videos are large, both of them have a size of almost 10 MByte!
.
Beware, the videos are large, both of them have a size of almost 10 MByte!
- Video 1: Start of reaction
- Video 2: Main part of reaction
After the reaction, a really dirty looking charred mess remains. This material has a strong smell of sulphur dioxide. It takes a long time (more than half an hour) before the black mass has completely cooled down.

![]()
Discussion of results
Concentrated sulphuric acid is both a strongly dehydrating agent, and an oxidizing agent. This combined action works on the sugar. The sugar is dehydrated, causing charring, but there also is partial oxidation, causing strong bubbling, due to formation of carbon dioxide and sulphur dioxide, which escape as gases. It is not possible to give a single simple chemical reaction equation, because there are many reactions, but the main gist of the reactions can be explained.
Plain sugar is a so-called disaccharide, with the following structure:
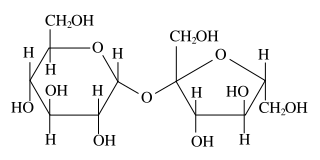
Its net formula is C12H22O11.
The dehydrating action of sulphuric acid, and the high temperature in the reaction mix causes the sugar to decompose. The decomposition of the sugar is a very complex process. A highly simplified reaction equation, which explains the main reaction products is the following:
C12H22O11 → 12C + 11H2O
The real reaction gives partially dehydrated compounds and a complex mix of organic compounds is produced, with carbon being the most extreme of the dehydrated compounds. Water, produced in the reaction, is absorbed by sulphuric acid.
Besides the dehydration, there is oxidation:
[C(H2O)m] + 2H2SO4 → CO2 + 2SO2 + (2+m)H2O
Here [C(H2O)m] stands for a complex partially dehydrated and charred sugar mix, which can be assumed to have an empirical formula of the form C(H2O)m, with m anything between 0 and 11/12.
The smoke in the reaction has a pungent smell of sulphur dioxide, mixed with a typical smell of charred sugar (smell of caramel).
This experiment also works with glucose or any other sugar. The type of reactions is similar with those sugars, in all cases, the sugar chars and is partially oxidized.