


Formation of a copper(III) complex
In aqueous solution, the chemistry of copper mostly is confined to the metal in the +2 oxidation state and the +1 oxidation state. The chemistry of the metal in the +2 oxidation state by far is the most extensive one. In this experiment, the metal is brought into the +3 oxidation state in a stable complex, which exists in aqueous solution, at high pH. The +3 oxidation state of copper really is remarkable and it is even more remarkable that this oxidation state is completely stable in aqueous solution.
![]()
![]() Required
chemicals:
Required
chemicals:
-
copper sulfate
-
potassium periodate
-
potassium hydroxide
-
sodium persulfate
![]() Required
equipment:
Required
equipment:
-
test tubes
-
an analytical balance makes the experiment more easy
![]() Safety:
Safety:
-
Potassium hydroxide is very corrosive. If this comes in contact with the skin, immediately rinse with a lot of water. Avoid contact with the eyes at any cost, a solution of potassium hydroxide is extremely destructive for the eyes.
-
Sodium persulfate and potassium periodate are strong oxidizers.
![]() Disposal:
Disposal:
-
The copper waste should not be flushed down the drain. Bring the waste to a proper waste processing facility.
![]()
Preparing the copper(III) complex
![]() Dissolve
40 mg of copper sulfate 5-hydrate in 3 ml of distilled water. This gives a pale
blue solution.
Dissolve
40 mg of copper sulfate 5-hydrate in 3 ml of distilled water. This gives a pale
blue solution.

![]() Add 200
mg of solid potassium periodate to this solution. If no balance is available,
then simply assure that an excess amount of potassium periodate is added. When
the potassium periodate is added, then a small part of this dissolves, and a
lime green precipitate of copper(II) metaperiodate is formed, but part of the
copper(II) also remains in solution.
Add 200
mg of solid potassium periodate to this solution. If no balance is available,
then simply assure that an excess amount of potassium periodate is added. When
the potassium periodate is added, then a small part of this dissolves, and a
lime green precipitate of copper(II) metaperiodate is formed, but part of the
copper(II) also remains in solution.

![]() In a
separate test tube, dissolve 200 mg of potassium hydroxide in 0.5 ml of water
and then add this solution to the test tube with the potassium periodate and the
lime green precipitate. If no balance is available, then prepare a concentrated
solution of potassium hydroxide and add this dropwise to the test tube, while
shaking, until all of the precipitate and all of the potassium periodate has
dissolved and a clear deep blue liquid is obtained.
In a
separate test tube, dissolve 200 mg of potassium hydroxide in 0.5 ml of water
and then add this solution to the test tube with the potassium periodate and the
lime green precipitate. If no balance is available, then prepare a concentrated
solution of potassium hydroxide and add this dropwise to the test tube, while
shaking, until all of the precipitate and all of the potassium periodate has
dissolved and a clear deep blue liquid is obtained.
Immediately after adding some potassium hydroxide, the test tube looks like shown in the left picture. When sufficient potassium hydroxide is added, and the test tube is shaken, then a clear deep blue solution is obtained, as shown in the right picture.


![]() Add 170
mg of sodium persulfate and carefully heat the test tube, until the liquid
starts boiling. When this is done, the color of the solution slowly goes from
blue to very dark red/brown through different shades of green and green/brown.
The color of the resulting liquid is very dark. The three pictures below show
the green colors, and the final dark red/brown liquid, which leaves a yellow
film on the glass. This adhering film of liquid demonstrates the intense color
of the solution.
Add 170
mg of sodium persulfate and carefully heat the test tube, until the liquid
starts boiling. When this is done, the color of the solution slowly goes from
blue to very dark red/brown through different shades of green and green/brown.
The color of the resulting liquid is very dark. The three pictures below show
the green colors, and the final dark red/brown liquid, which leaves a yellow
film on the glass. This adhering film of liquid demonstrates the intense color
of the solution.



![]() Take two
drops of the intensely dark red/brown liquid and add a lot of water. When this
is done, a nice red solution is obtained.
Take two
drops of the intensely dark red/brown liquid and add a lot of water. When this
is done, a nice red solution is obtained.

It really is remarkable how intense the color of this red/brown complex of copper(III) is, given the pale color of the starting solution.
![]() When the dark red/brown solution is allowed to stand for a
few days, then a nice crystalline mass settles. After a few hours, a colorless
crystal mass settles, but after a few days, other crystals of a different shape
and color settle at the bottom as well. The liquid itself becomes much lighter,
comparable to the liquid shown above. The dark crystals are shown in the picture
below.
When the dark red/brown solution is allowed to stand for a
few days, then a nice crystalline mass settles. After a few hours, a colorless
crystal mass settles, but after a few days, other crystals of a different shape
and color settle at the bottom as well. The liquid itself becomes much lighter,
comparable to the liquid shown above. The dark crystals are shown in the picture
below.

The liquid was decanted from the crystal mass and the solid was allowed to dry for a few days in contact with air. After this period, the solids look as follows:

The yellow material is a colorless solid, contaminated with some of the copper(III) complex, the almost black crystal mass is the copper(III) complex, crystallized with potassium or sodium cation.
When the test tube is allowed to dry for several days, then the dark crystal mass becomes really dry and it can easily be separated from the light crystal mass, simply by mechanical means. Only approximately 25% of the dark crystals stick to the light crystals and those are discarded. The remaining dark crystal mass was isolated and put in a separate vial.
A second experiment was done, with somewhat more copper sulfate and more potassium iodate, in order to get a little more of the dark solid. The second batch again resulted in easy to separate dark crystals.
A picture is made from the vial, in bright sunshine, while the vial is placed on a black surface. Under these conditions, it is clearly visible that the crystals are very dark red. When viewed in dim light, or under tungsten light, then the crystals simply look black, only the bright direct sunlight reveals the red color.

When these crystals are added to water, then they dissolve very slowly, giving a clear yellow/brown solution, which at higher concentration is red/brown.
![]()
Discussion of the results
When copper(II) ions and periodate ions are present at sufficiently high pH, then the following complex ion is produced:
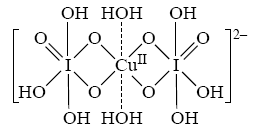
Two orthoperiodate ions are coordinated to a copper(II) ion, and besides these, two water molecules are coordinated loosely. This complex has a very deep blue color. Even dilute solutions of this complex are distinctly blue.
This complex can be oxidized in strongly alkaline environments. The loosely coupled water ligands under such conditions are detached. Two hydroxide ligands firmly attach to the central copper atom and at the same time an electron is transferred from the central copper atom. The resulting complex then is
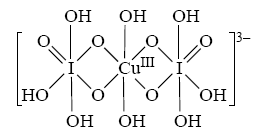
This complex has a very deep brown/red color.
The net reaction equation is
![]()
Here, the loosely coupled water molecules are not taken into account.
The solid material most likely is K3[Cu(H3IO6)2(OH)2], potassium bis-orthoperiodato dihydroxo cuprate(III).
Remark: The information about this complex is obtained from the following paper:
Complexes of Copper in Unstable Oxidation States, Russian Journal of Coordination Chemistry, Vol. 29, No. 11, 2003, pp. 743–765.
This paper is a translation from the original Russion text in:
Koordinatsionnaya Khimiya, Vol. 29, No. 11, 2003, pp. 803–827. Original Russian text Copyright © 2003 by T. V. Popova and N. V. Aksenova.
According to the paper, mentioned above, similar complexes also exist for copper(III) with tellurate(VI) ions.