


Reaction between aluminium and bromine
Bromine and aluminium react very violently with each other, when they are brought in contact with each other. The reaction, however, is not immediate. There is an induction period of well over a minute, before the reaction really starts, but once it is going, it is very violent and spectacular.

With only aluminium metal and bromine, the reaction proceeds with fire-like appearance. When a drop of water is added, then the reaction also proceeds violently, with production of immense amounts of thick fumes of HBr and AlBr3. In that case, the reaction is as spectacular as without the drop of water. The amount of water, however, should be relatively small. Too much water quenches the reaction.

 This is a spectacular, but also quite dangerous experiment. Be very careful and
never scale up the experiment for added effect! Also assure that the
fumes and smoke, produced in this reaction, are not breathed. These fumes and
smoke are intensely corrosive and very poisonous. This experiment MUST be
performed in a fume hood, or outside.
This is a spectacular, but also quite dangerous experiment. Be very careful and
never scale up the experiment for added effect! Also assure that the
fumes and smoke, produced in this reaction, are not breathed. These fumes and
smoke are intensely corrosive and very poisonous. This experiment MUST be
performed in a fume hood, or outside.
![]()
![]() Required
chemicals:
Required
chemicals:
-
bromine
-
aluminium foil
-
(dilute hydrochloric acid and sodium sulfite for safe disposal)
![]() Required
equipment:
Required
equipment:
-
test tube
- a safe way to attach the test tube firmly, do not hold it in your hand.
- a dropper, needed for transferring the bromine from its container to the test tube
![]() Safety:
Safety:
-
 Bromine is very
toxic. The liquid causes deep and painful wounds, when it is spilled on the
skin. The vapor of bromine is very corrosive and highly toxic. The smell of
bromine is terrible.
Bromine is very
toxic. The liquid causes deep and painful wounds, when it is spilled on the
skin. The vapor of bromine is very corrosive and highly toxic. The smell of
bromine is terrible. -
 The reaction
is very violent and a lot of corrosive and toxic fumes are produced. Assure
that the reaction is performed in a VERY well ventilated room, or outside.
The reaction
is very violent and a lot of corrosive and toxic fumes are produced. Assure
that the reaction is performed in a VERY well ventilated room, or outside.
![]() Disposal:
Disposal:
- After the reaction, add some dilute hydrochloric acid to dissolve remains of metal and then add some sodium sulfite for destroying remains of bromine. The final waste can be flushed down the drain with lots of water. The waste is not particularly toxic.
![]()


Aluminium with dry bromine
![]() Take a piece of aluminium foil and put this in a test tube.
The two pictures below show the foil in the test tube. A piece of foil of
approximately 3 cm length and 5 mm width is used.
Take a piece of aluminium foil and put this in a test tube.
The two pictures below show the foil in the test tube. A piece of foil of
approximately 3 cm length and 5 mm width is used.


![]() Add approximately 0.3 ml of liquid bromine. Use a small dropper to add the
bromine to the test tube. Be careful here.
Add approximately 0.3 ml of liquid bromine. Use a small dropper to add the
bromine to the test tube. Be careful here.
![]() Bromine is a very
mobile and dense liquid and it is really easy to spill some drops of bromine.
Never allow any liquid bromine to touch your skin. Have a bottle of solution
of sodium thiosulfate ready to grasp, when accidently some bromine is spilled on
the skin.
Bromine is a very
mobile and dense liquid and it is really easy to spill some drops of bromine.
Never allow any liquid bromine to touch your skin. Have a bottle of solution
of sodium thiosulfate ready to grasp, when accidently some bromine is spilled on
the skin.
The following two pictures show the test tube with the aluminium foil, to which the small amount of bromine is added.


Now, at first nothing seems to happen. The bromine vapor very slowly escapes from the test tube, but besides that, there are no visible changes.
After two minutes or so, however, suddenly the bromine starts developing a thick vapor and then things go fast. The aluminium foil starts 'burning' in the thick vapor above the liquid bromine. The 'flames' become quite large and at the moment that the reaction is most violent, the 'flame' has a size of almost 2 cm. The color of the 'flame' is deep orange/red. Probably this deep color is due to the fact that the 'flame' is observed through a layer of dense bromine vapor. The aluminium foil is moving around in the test tube over the surface of the bromine, in the meantime 'burning'. The following three pictures show these phenomena. Click on a picture for a full-size picture for more detail.
A heavy and dense fume, consisting of a mix of aluminium bromide and bromine is flowing over the rim of the test tube. It is remarkable to see how this fume goes downwards quickly. It only extends a few mm above the rim of the test tube, in spite of the high temperature of the fume. The left picture nicely shows this.
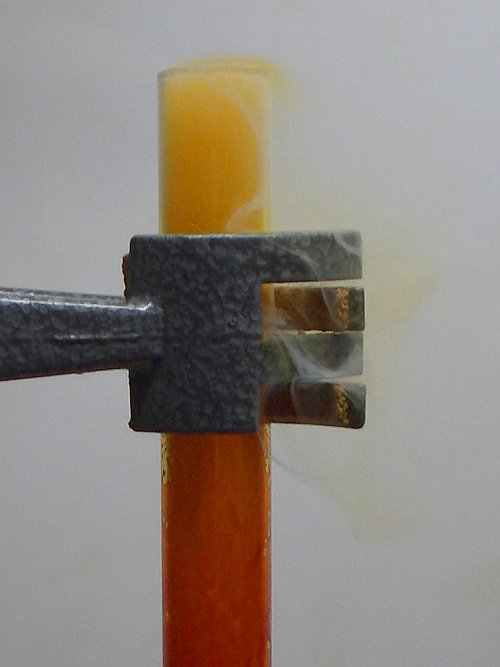

The right picture shows a close-up of the 'flame' of the 'burning' aluminium, above the liquid bromine.
The aluminium keeps on burning for approximately 30 seconds. After that, the aluminium is totally gone and only the remaining liquid bromine can be observed. The aluminium completely is converted to aluminium bromide and that is present as white 'frost' near the rim of the test tube. The picture below shows the test tube, immediately after the 'flame' disappeared again. One can nicely see the condensing of warm bromine vapor on the cooler glass.

A video was made of this reaction as well: video of reaction of Al and Br2. Beware, download size of this video is approximately 6.3 MByte, but the video is spectacular.
At the beginning, just before the reaction sets in, and just after adding the drops of bromine, 1 minute of video data was cut. The reaction, shown on the video had an induction period of more than 1 minute, and that was cut from the video.
![]()
Aluminium with wet bromine
The same experiment is repeated, but now with a single large drop of water added to the bromine, before aluminium is added. After the previous experiment, there still is sufficient bromine in the test tube to perform this experiment, so no new bromine needs to be added.
![]() To the
test tube, resulting as the end of the previous experiment, add a single large
drop of water with a dropper. This results in a fairly violent reaction between
the anhydrous aluminium bromide, apparently immersed in the bromine, and the
water. Some roaring noise can be observed for a second or so. Be sure to add
just a single drop of water. Adding too much water makes the bromine too dilute
and then the experiment will not succeed.
To the
test tube, resulting as the end of the previous experiment, add a single large
drop of water with a dropper. This results in a fairly violent reaction between
the anhydrous aluminium bromide, apparently immersed in the bromine, and the
water. Some roaring noise can be observed for a second or so. Be sure to add
just a single drop of water. Adding too much water makes the bromine too dilute
and then the experiment will not succeed.
![]() To the bromine/water mix, again add a piece of aluminium
foil, similar in size, used in the previous experiment.
To the bromine/water mix, again add a piece of aluminium
foil, similar in size, used in the previous experiment.
At the aluminium, a fairly violent fizzling can be observed. There are no signs of fire. The temperature, however, rises considerably and the test tube becomes very hot again. What is more noticeable is that a lot of gas is produced and an intensely thick fume is produced as well. The amount of fume, produced in this experiment, really is spectacular and much more than in the previous experiment without water. The following picture shows the result, approximately 20 seconds after adding the aluminium foil to the bromine/water mix.

The fume/smoke is very heavy and it really flows like a liquid out of the test tube and it falls downward, along the test tube, and it forms a white frost on the clamp, which holds the test tube. As the picture at the right shows, the fume only rises a few mm above the rim of the test tube and it 'falls' down almost vertically. It really is spectacular to watch this happen.

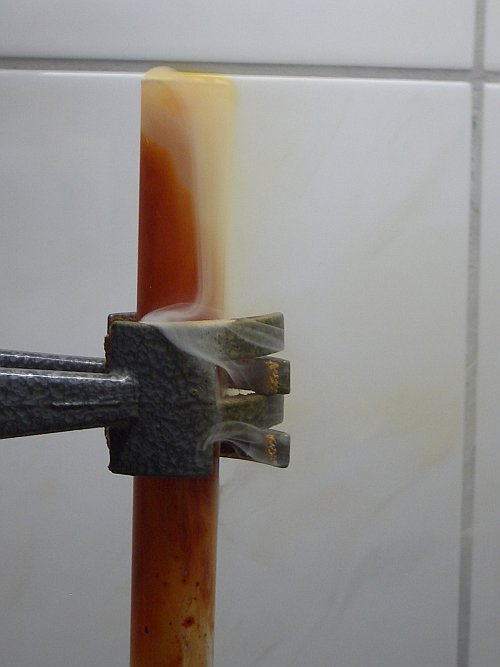
It really is remarkable to get such a large amount of fume from such a small amount of bromine, water and aluminium metal!
When the reaction is done, then the test tube is covered with a thick light-yellow frost-like layer of aluminium bromide, contaminated with traces of bromine, water and hydrobromic acid. The thick fume for a large part consists of hydrobromic acid (HBr). The clamp, holding the test tube also is covered by the light-yellow 'frost'. The test tube itself is filled with a brown mix of water, bromine and aluminium bromide, with small particles of unreacted aluminium metal remaining. This is shown in the right picture.


Even minutes after the reaction has subsided, there still is quite some heavy fume in the test tube. As long as it is kept vertically, no fume escapes from the test tube. If it is kept horizontally, then the fume can be 'poured' out of the test tube. The fume still is heavy and most of it moves downward, as is shown by the following picture.

![]() To the
the test tube add several ml of water.
To the
the test tube add several ml of water.

Adding the water makes the entire test tube fill up with a thick fume again and the liquid becomes yellow and turbid. The turbidity is due to fairly large amounts of aluminium hydroxide, formed in the reaction, described above.
In the reaction with bromine and water a lot of heat is produced and this causes the aluminium bromide and water to react, forming aluminium hydroxide or basic aluminium bromide, together with hydrobromic acid, which escapes as thick fume. What remains in the test tube is aluminium hydroxide with remains of bromine and metal, together with aluminium bromide. On addition of much more water this causes the liquid to become turbid and yellow.
![]()
Discussion of results
![]() Aluminium and bromine react with each other, producing aluminium bromide and a
lot of heat:
Aluminium and bromine react with each other, producing aluminium bromide and a
lot of heat:
2Al + 3Br2 → 2AlBr3
The induction time probably is due to the oxide layer of the aluminium. It takes some time, before the bromine has found its way through the oxide layer and during that time no visible reaction occurs.
The compound AlBr3 is a fairly volatile covalent compound. This explains the white fumes, mixed with the bromine vapor, escaping from the test tube in the experiment with dry bromine.
![]() When a drop of water is added to the test tube, then some roaring noise can be
observed. This is due to the violent reaction between anhydrous AlBr3
and water. The covalent compound AlBr3 is converted to an ionic
compound (a salt) AlBr3.6H2O. Better notation is [Al(H2O)6]Br3.
Besides that, there also will be quite some hydrolysis, resulting in formation
of basic compounds of aluminium and hydrobromic acid, which escapes as gas.
When a drop of water is added to the test tube, then some roaring noise can be
observed. This is due to the violent reaction between anhydrous AlBr3
and water. The covalent compound AlBr3 is converted to an ionic
compound (a salt) AlBr3.6H2O. Better notation is [Al(H2O)6]Br3.
Besides that, there also will be quite some hydrolysis, resulting in formation
of basic compounds of aluminium and hydrobromic acid, which escapes as gas.
![]() When
aluminium is added to the water/bromine mix, then
again aluminium bromide is formed, but there now also is reaction with the
water, accompanied with extensive hydrolysis at the high temperatures involved
and the small amounts of water involved, allowing the HBr to escape as gas.
When
aluminium is added to the water/bromine mix, then
again aluminium bromide is formed, but there now also is reaction with the
water, accompanied with extensive hydrolysis at the high temperatures involved
and the small amounts of water involved, allowing the HBr to escape as gas.
AlBr3 + 3H2O → 2Al(OH)3 + 3HBr
The HBr gives rise to formation of the immense amounts of fume. In the air, it fumes strongly. Together with remaining anhydrous AlBr3 and some bromine vapor the fumes look remarkably thick.


