


Chlorine
 The element chlorine is a very abundant element, which is
encountered in many compounds, known from everyday life. The element itself,
however, is much less common for the general public. It is a fairly pale green/yellow
gas, which is very toxic, when inhaled. Luckily, it has a strong choking odor.
This provides a good warning for potentially dangerous situations, when one is
working with chlorine gas.
The element chlorine is a very abundant element, which is
encountered in many compounds, known from everyday life. The element itself,
however, is much less common for the general public. It is a fairly pale green/yellow
gas, which is very toxic, when inhaled. Luckily, it has a strong choking odor.
This provides a good warning for potentially dangerous situations, when one is
working with chlorine gas.
![]() Experiments with
chlorine gas should not be conducted inside, unless one has a good fume hood. A
standard kitchen exhaust is not sufficient for working with chlorine.
Experiments with
chlorine gas should not be conducted inside, unless one has a good fume hood. A
standard kitchen exhaust is not sufficient for working with chlorine.
Chlorine can easily be prepared with common chemicals, but that must be done with care, due to its toxicity. It is a very reactive chemical, which can be used in many interesting experiments. On this site there are several experiments, in which elementary chlorine is used. This chlorine is made, when it is needed. One should not store chlorine gas! It is very corrosive and almost certainly escapes from its container within a few days and will corrode everything, stored nearby.
![]()
Chlorine exists in many oxidation states, being -1, 0, +1, +3, +4, +5, +7. The most stable chlorine compounds are the ones with oxidation state -1, the chlorides. Oxidation state +7 also is remarkably stable in inorganic perchlorates. The following inorganic compounds are available for the home chemist. All of them are quite interesting.
- sodium chloride, NaCl
- potassium chloride, KCl
- hydrogen chloride, hydrochloric acid, aqueous HCl
- sodium hypochlorite, bleach, NaOCl
- calcium hypochlorite, Ca(ClO)2
- sodium chlorate, NaClO3
- potassium chlorate, KClO3
- potassium perchlorate, KClO4
More chlorides are available, but these are not covered here explicitly. These chlorides are compounds, where the main property of the compound is determined by the cation and the chloride ion is chosen as a relatively inert counter ion.
![]() Sodium chloride is the well known table salt and potassium chloride is an
alternative for sodium chloride, used by people who need a low-sodium diet.
These salts are not particularly reactive nor corrosive. They can be used safely
in chemical experiments. For some experiments, the use of potassium chloride may
be advantageous, but usually these two can be exchanged. One of these two should
be present in the home lab, also because they can be obtained easily at high
purity.
Sodium chloride is the well known table salt and potassium chloride is an
alternative for sodium chloride, used by people who need a low-sodium diet.
These salts are not particularly reactive nor corrosive. They can be used safely
in chemical experiments. For some experiments, the use of potassium chloride may
be advantageous, but usually these two can be exchanged. One of these two should
be present in the home lab, also because they can be obtained easily at high
purity.
![]() Hydrogen chloride is a colorless gas, which dissolves very well in water. It is
not sold as the gas, but as a solution in water. This solution is strongly
acidic. The technical grade solution at a concentration of approximately 30% HCl
is called muriatic acid, the chemical name for the solution is hydrochloric
acid. Muriatic acid can be light green or yellow. This color is due to
impurities, being iron-compounds or organic compounds. A pure solution of
hydrogen chloride in water is colorless. Hydrochloric acid can be used for many
purposes, especially when a cheap, easy to obtain non-oxidizing acid is needed.
Hydrogen chloride is a colorless gas, which dissolves very well in water. It is
not sold as the gas, but as a solution in water. This solution is strongly
acidic. The technical grade solution at a concentration of approximately 30% HCl
is called muriatic acid, the chemical name for the solution is hydrochloric
acid. Muriatic acid can be light green or yellow. This color is due to
impurities, being iron-compounds or organic compounds. A pure solution of
hydrogen chloride in water is colorless. Hydrochloric acid can be used for many
purposes, especially when a cheap, easy to obtain non-oxidizing acid is needed.
A well equipped home lab should have some hydrochloric acid present. For many purposes muriatic acid can be used, but sometimes it is best to use the colorless grade of the acid. Muriatic acid can be purchased at some hardware stores. In most hardware stores (at least in the Netherlands) also dilute hydrochloric acid (approximately 10% HCl by weight) can be purchased. Some brands of this dilute acid are very clean and are completely colorless. For many experiments this is preferred over the yellow muriatic acid. When the acid is used for producing chlorine gas with hypochlorite, then the yellow impurity is not of any concern.
![]() Concentrated
hydrochloric acid (30 - 37% of HCl by weight) fumes in air. These fumes are
highly irritating and should not be inhaled. Dilute acid with less than 15% of
HCl does not fume when it is at room temperature or below. When the acid is
heated, then the fumes may become very dense and really choking. Such
experiments must be performed outside.
Concentrated
hydrochloric acid (30 - 37% of HCl by weight) fumes in air. These fumes are
highly irritating and should not be inhaled. Dilute acid with less than 15% of
HCl does not fume when it is at room temperature or below. When the acid is
heated, then the fumes may become very dense and really choking. Such
experiments must be performed outside.
Hydrochloric acid is corrosive. The fumes of the concentrated acid also are very corrosive. A bottle of concentrated acid, stored near an iron part causes the iron to rust much faster than it does under normal conditions!
![]() Sodium hypochlorite only is stable in alkaline aqueous solution. The most
concentrated form one can obtain contains 15% active chlorine. Common household
bleach contains 4 or 5% of active chlorine. When this compound is used in
chemical experiments, it is important to use the cheap stuff, which does not
contain any perfume, surfactants and compounds to make the liquid viscous. Plain
bleach is a mobile colorless or very light yellow liquid with a slight
'chlorine'-odor. Common household bleach can be obtained in supermarkets, the
more concentrated liquid can be obtained in stores, where swimming pool
additives are sold.
Sodium hypochlorite only is stable in alkaline aqueous solution. The most
concentrated form one can obtain contains 15% active chlorine. Common household
bleach contains 4 or 5% of active chlorine. When this compound is used in
chemical experiments, it is important to use the cheap stuff, which does not
contain any perfume, surfactants and compounds to make the liquid viscous. Plain
bleach is a mobile colorless or very light yellow liquid with a slight
'chlorine'-odor. Common household bleach can be obtained in supermarkets, the
more concentrated liquid can be obtained in stores, where swimming pool
additives are sold.
Sodium hypochlorite can be used for many redox experiments in alkaline environments. It is a remarkable strong oxidizer. It is even capable of oxidizing manganese to its +7 oxidation state and iron to its +6 oxidation state. This is demonstrated in a nice experiment.
![]() Although bleach is a very common chemical in the household, its dangers should
not be underestimated. It is a highly corrosive liquid, especially for the eyes.
With acids, very toxic chlorine gas is evolved.
Although bleach is a very common chemical in the household, its dangers should
not be underestimated. It is a highly corrosive liquid, especially for the eyes.
With acids, very toxic chlorine gas is evolved.
![]() Calcium hypochlorite also is stable in the solid form. It is available as an
off-white powder, which contains 65% - 70% of active chlorine, meaning that more
than 90% of the solid consists of calcium hypochlorite. Usually the commercial
product also contains some calcium chloride, calcium hydroxide and calcium
carbonate. A well-known brand of this is so called HTH calcium hypochlorite,
which is available in shops, which sell swimming pool additives. Calcium
hypochlorite does not dissolve cleanly in water, it rather forms a white slurry.
On addition of hydrochloric acid, a lot of chlorine gas is produced. This
compound can be used as a convenient source of fairly pure chlorine gas in many
experiments.
Calcium hypochlorite also is stable in the solid form. It is available as an
off-white powder, which contains 65% - 70% of active chlorine, meaning that more
than 90% of the solid consists of calcium hypochlorite. Usually the commercial
product also contains some calcium chloride, calcium hydroxide and calcium
carbonate. A well-known brand of this is so called HTH calcium hypochlorite,
which is available in shops, which sell swimming pool additives. Calcium
hypochlorite does not dissolve cleanly in water, it rather forms a white slurry.
On addition of hydrochloric acid, a lot of chlorine gas is produced. This
compound can be used as a convenient source of fairly pure chlorine gas in many
experiments.
![]() Calcium hypochlorite is a fairly dangerous chemical. It is a strong
oxidizer, which reacts quickly, sometimes violently. With many organic
compounds, sensitive mixtures can be formed, which can ignite easily.
Calcium hypochlorite is a fairly dangerous chemical. It is a strong
oxidizer, which reacts quickly, sometimes violently. With many organic
compounds, sensitive mixtures can be formed, which can ignite easily.
![]() Calcium hypochlorite slowly gives off chlorine gas. The material should only be
stored in its original container in a well ventilated place.
Calcium hypochlorite slowly gives off chlorine gas. The material should only be
stored in its original container in a well ventilated place.
![]() Sodium chlorate and potassium chlorate are strong oxidizers. Sodium chlorate is
a white to light yellow solid, potassium chlorate is a white crystalline powder.
The solution of these compounds in water reacts somewhat sluggish, but in the
solid state, these oxidizers can react violently. Sodium chlorate dissolves in
water very well, potassium chlorate dissolves with much more difficulty. If one
wants to use chlorates for aqueous chemistry experiments, then sodium chlorate
definitely is the preferred choice. For dry-chemical experiments, potassium
chlorate is the preferred choice. The sodium compound is quite hygroscopic.
Sodium chlorate and potassium chlorate are strong oxidizers. Sodium chlorate is
a white to light yellow solid, potassium chlorate is a white crystalline powder.
The solution of these compounds in water reacts somewhat sluggish, but in the
solid state, these oxidizers can react violently. Sodium chlorate dissolves in
water very well, potassium chlorate dissolves with much more difficulty. If one
wants to use chlorates for aqueous chemistry experiments, then sodium chlorate
definitely is the preferred choice. For dry-chemical experiments, potassium
chlorate is the preferred choice. The sodium compound is quite hygroscopic.
The redox chemistry of chlorates is complex. They can be reduced to chlorine dioxide, an intensely yellow colored gas, they can be reduced to chlorine or to chloride. The exact reaction product depends on the experimental conditions. Unfortunately, chlorate compounds cannot be used for clean redox experiments. There hardly are any experimental conditions, which yield only one reaction product. Usually a complex mixture of different chlorine-compounds is formed. Click here for an experiment, in which chlorine dioxide is produced by reducing sodium chlorate with hydrochloric acid.


![]() Mixtures of chlorates with reducing agents, such as dry wood, sugar, sulphur,
carbon and metal powders, are dangerous and very sensitive. Unless you know
what you are doing, do not store such mixtures! Sodium chlorate and
potassium chlorate can be used in dry-chemical experiments, but these
experiments are dangerous and should only be carried out with sub-gram
quantities, outside and in a non-confined space. Chlorate mixtures are so
sensitive, that they can ignite spontaneously, apparently without reason.
Never grind mixtures of chlorates and reducing agents. If one of the
compounds needs to be ground, then this must be done before mixing.
Mixtures of chlorates with reducing agents, such as dry wood, sugar, sulphur,
carbon and metal powders, are dangerous and very sensitive. Unless you know
what you are doing, do not store such mixtures! Sodium chlorate and
potassium chlorate can be used in dry-chemical experiments, but these
experiments are dangerous and should only be carried out with sub-gram
quantities, outside and in a non-confined space. Chlorate mixtures are so
sensitive, that they can ignite spontaneously, apparently without reason.
Never grind mixtures of chlorates and reducing agents. If one of the
compounds needs to be ground, then this must be done before mixing.
Potassium chlorate can be obtained at pyrotechnics suppliers, some photography raw chemical suppliers and chemical supply houses. Sodium chlorate sometimes can be purchased as a herbicide, but the places, where it is sold as such are quickly disappearing. For most people the only source probably will be chemical supply houses.
Potassium chlorate and sodium chlorate are nice additions to the home lab for the cautious user. For people without much experimental experience and with limited knowledge of chemistry it is better to steer away from chlorates, at least for the time being.
![]() Potassium perchlorate also is a strong oxidizer, almost as strong as chlorates.
It is a white crystalline solid. Aqueous solutions of potassium perchlorate,
however, are remarkably inert. Only at high concentrations of acid, the perchlorate ion tends to
react in aqueous solution. Combining this property with the low solubility of
potassium perchlorate in water, it can be concluded that this chemical is not
really interesting for the home lab, when one performs aqueous chemistry
experiments only.
Potassium perchlorate also is a strong oxidizer, almost as strong as chlorates.
It is a white crystalline solid. Aqueous solutions of potassium perchlorate,
however, are remarkably inert. Only at high concentrations of acid, the perchlorate ion tends to
react in aqueous solution. Combining this property with the low solubility of
potassium perchlorate in water, it can be concluded that this chemical is not
really interesting for the home lab, when one performs aqueous chemistry
experiments only.
Certain dry-chemical experiments can be performed with potassium perchlorate, just as with potassium chlorate. Mixtures with potassium perchlorate are less sensitive than mixtures with potassium chlorate, so in general it is safer to perform dry chemical experiments with potassium perchlorate.
Potassium perchlorate is hard to obtain, except possibly through certain pyrotechnics suppliers. The only other source probably will be chemical supply houses.
![]()
There are two organic compounds of chlorine, which are particularly interesting for the home chemist, and which are easy to obtain at high purity and which are cheap. Both are used as organic swimming pool chlorinating agents, for use in outside swimming pools. One of them is used as shock-treatment, and the other is used for slow-release chlorinating. These chemicals are sodium dichloroisocyanurate (shock treatment) and trichloroisocyanuric acid (slow release). The chemical structure of these compounds is as follows:
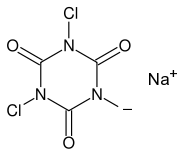
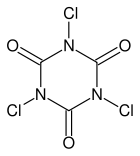
Both of them are very strong oxidizers, capable of making copious amounts of really pure chlorine, simply by adding some dilute hydrochloric acid. These are chemicals, which should be present in every home lab, where some serious experiments are performed with halogens. They are available from many gardening and hobby stores, and from swimming pool stores.
These two chemicals do not belong to the class of so-called chlorinated hydrocarbons. There are no direct carbon-chlorine bonds in these chemicals, and the chlorine is released easily. These are not toxic and carcinogenic chemicals, but of course, they should be treated with respect, due to their oxidizing power and the possible release of copious amounts of chlorine, when acid is added.
![]()
Besides the inorganic compounds and the two organic compounds, mentioned above, there are numerous other organic chlorine compounds, available to the general public. Some of these are:
- tetrachloromethane, tetra, CCl4
- trichloromethane, chloroform, CHCl3
- dichloromethane, methylene chloride, CH2Cl2
- trichloroethylene, tri, CHClCCl2
- tetrachloroethylene, per, CCl2CCl2
- 1,1,1-trichloroethane, solventane, CCl3CH3
- para-dichlorobenzene, moth balls, ClC6H4Cl
In the list, first the chemical formal name is given, then a common name and then the formula.
![]()
![]() Many chlorinated
hydrocarbons are carcinogens. Especially tetrachloromethane is notoriously toxic
and an established cancer causing chemical. The use of chlorinated hydrocarbons
must be avoided as much as possible. For the home lab, these chemicals only are
of limited interest.
Many chlorinated
hydrocarbons are carcinogens. Especially tetrachloromethane is notoriously toxic
and an established cancer causing chemical. The use of chlorinated hydrocarbons
must be avoided as much as possible. For the home lab, these chemicals only are
of limited interest.
All of these chemicals are covalent, volatile compounds. The first compounds all are colorless liquids, the latter is a low-melting white crystalline solid.