


Aqueous solutions and precipitates of manganese
In aqueous solutions, manganese is said to exist in the +2, +3, +4, +5, +6 and +7 oxidation states. In my own experience, all of these oxidation states can be achieved, except oxidation state +5. So, if oxidation state +5 can exist in aqueous solution, then it is very unstable and really hard to obtain. In this page, unfortunately no pictures can be provided of the +5 oxidation state.
Oxidation state +2 is easy to achieve and is very stable in acidic and neutral media. In alkaline media, it is stable, but very prone to aerial oxidation. This is demonstrated nicely below. Oxidation state +3 is quite unstable. It only exists at very low pH (around 0), at higher pH it tends to hydrolyse, forming a brown precipitate of hydrous Mn2O3. Oxidation state +4 only is feasible in concentrated hydrochloric acid, probably as a manganese (IV) chloro complex. On dilution of the acid, the manganese in the +4 oxidation state hydrolyses to dark brown hydrous MnO2. Manganese in its +6 oxidation state is only stable in strongly alkaline solutions (pH equal to 13 and upwards) as the MnO42- ion, but at such high pH it can be achieved very easily. Finally, manganese in its +7 oxidation state is stable over the entire acidic side of the pH scale and still fairly stable in alkaline solutions, as long as the alkalinity is not too strong. E.g. at pH equal to 11, a purple solution of permanganate remains purple for a sufficiently long time, such that it can be handled without troubles, however at pH > 14, a solution of a permanganate quickly turns green and the permanganate decomposes, loosing oxygen.
![]()
Oxidation state +2
This oxidation state is the most stable one in acidic and neutral aqueous environments. Many textbooks describe the aqueous manganese (II) ion as light pink, but I would say it is colorless. The picture below shows a fairly concentrated solution of manganese (II) sulfate and it looks completely colorless. Compare this with the picture of solid manganese (II) sulfate. The latter also has a very faint pink color.


 Although
manganese (II) is the most stable in acidic and neutral environments, it is very
hard to obtain in the pure state in alkaline environments. A precipitate of
manganese (II) hydroxide is white, but it quickly
turns brown, due to aerial oxidation, forming manganese (III) hydroxide or
manganese (III) oxide/hydroxide. The picture shows a precipitate of manganese
(II) hydroxide, which was allowed to have contact with air for several seconds.
The picture clearly shows the brown color, due to aerial oxidation and the
precipitate also already is slightly yellow, due to oxidation. In fact, without
special equipment, allowing one to work in an environment, totally free of air,
it is virtually impossible to prepare a really white precipitate of Mn(OH)2.
This picture was made, by adding a tiny amount of NaBH4 to the
solution of NaOH, to which the solution of manganese (II) sulfate was added.
Otherwise, making a picture like this would not be at all possible, because of
the immediate formation of a brown precipitate, due to dissolved oxygen in the
water. The NaBH4 takes out most of the oxygen, dissolved in water.
Although
manganese (II) is the most stable in acidic and neutral environments, it is very
hard to obtain in the pure state in alkaline environments. A precipitate of
manganese (II) hydroxide is white, but it quickly
turns brown, due to aerial oxidation, forming manganese (III) hydroxide or
manganese (III) oxide/hydroxide. The picture shows a precipitate of manganese
(II) hydroxide, which was allowed to have contact with air for several seconds.
The picture clearly shows the brown color, due to aerial oxidation and the
precipitate also already is slightly yellow, due to oxidation. In fact, without
special equipment, allowing one to work in an environment, totally free of air,
it is virtually impossible to prepare a really white precipitate of Mn(OH)2.
This picture was made, by adding a tiny amount of NaBH4 to the
solution of NaOH, to which the solution of manganese (II) sulfate was added.
Otherwise, making a picture like this would not be at all possible, because of
the immediate formation of a brown precipitate, due to dissolved oxygen in the
water. The NaBH4 takes out most of the oxygen, dissolved in water.
![]()
Oxidation state +3
Oxidation state +3 is only stable in strongly acidic media, with a pH around 0, or even lower. Aqueous manganese (III) ions have a nice pink/brown color, as is shown on the picture below. When such a solution is added to a solution of sodium hydroxide, then a brown hydrous precipitate of Mn(OH)3 or MnO(OH) is produced. If a solution, containing Mn3+(aq) is diluted, or when the pH is brought up well above 0, then the liquid slowly becomes turbid and a brown precipitate of Mn(OH)3 or MnO(OH) is produced.
The two pictures below show
- a highly acidic solution containing Mn3+(aq)
- a precipitate of Mn(OH)3 or MnO(OH).
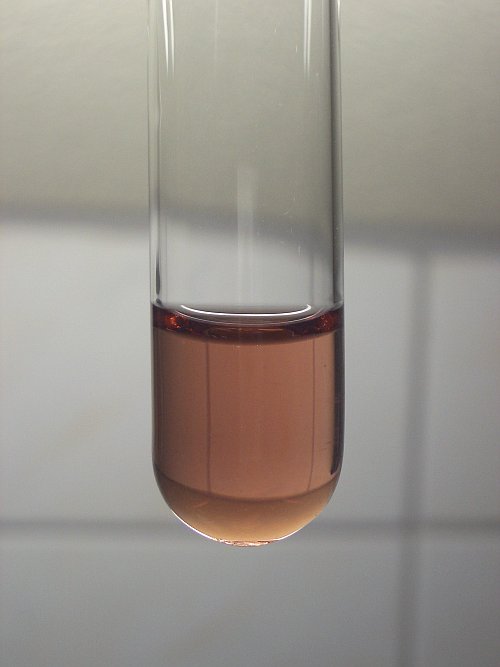

![]()
Oxidation state +4
Oxidation state +4 is the most stable one in the solid state, but only in the form of its oxide MnO2. The oxide MnO2 is a black or dark gray solid, absolutely insoluble in water. In fact, the only really well known compound of manganese in its +4 oxidation state is MnO2, and even then, it is notoriously difficult to have all of the manganese in oxidation state +4. Even the best quality samples of MnO2 are slightly oxygen-deficient and can better be described as MnO2-x, with x in the order of magnitude of 0.05.
A remarkable observation is that when some potassium permanganate or hydrous manganese dioxide is added to cool concentrated hydrochloric acid, then a dark brown/green compound is formed. With MnO2 as the starting point, initially hardly any chlorine gas can be smelled and all solid dissolves, forming a brown/green clear solution. With KMnO4 as the starting point, some chlorine is formed, but the manganese (VII) is not reduced all the way down to manganese (II), but to the same dark brown/green compound, as what is formed on adding MnO2 to concentrated HCl.
Based on the above mentioned observations, in aqueous solution, the +4 oxidation state seems to be fairly stable in highly concentrated hydrochloric acid (not in sulphuric acid or nitric acid of similar normality). Probably, the concentrated chloride forms a chloro-complex of manganese (IV), which is very prone to hydrolysis and which also is strongly oxidizing. So, concentrated hydrochloric acid, containing manganese (IV) slowly looses its color, the manganese being reduced to the colorless Mn2+ ions and the chloride being oxidized to Cl2.
Below, some concentrated hydrochloric acid (30% HCl by weight) is shown, which contains a very small amount of the brown/green species. Only a few hundreds of micrograms of manganese are present in this solution, representing a dilution in the order of magnitude of 1 : 10000. The color of this compound is very strong. The color is dirty green/brown. When the solution is made less acidic, by adding some hydroxide, then a very voluminous dark brown precipitate of hydrous MnO2 is formed. This is shown in the right picture.


The precise composition of this dark brown/green compound is not known to me. Some people think it is a manganese (IV) chloro complex, some others think it is a mixed valency complex of manganese (III) and manganese (IV). Some textbooks also mention the existence of a complex ion MnCl52-, but when this were formed, then immediately, quite some chlorine gas would be expected on mixing MnO2 and cold concentrated hydrochloric acid, but this is not the case. The MnO2 dissolves and initially no chlorine can be observed.
![]()
Oxidation state +6
This oxidation state only is stable in strongly alkaline environments. Manganese in the +6 oxidation state can easily be prepared by adding a very concentrated solution of sodium hydroxide or potassium hydroxide to a solution of potassium permanganate. The permanganate decomposes under such strongly alkaline conditions, giving oxygen and dark green manganate:
4MnO4– + 4OH– → 4MnO42– + O2 + 2H2O
The following picture shows the manganate and the small bubbles of oxygen, formed from the decomposition of the permanganate. The color of manganate also is very strong and it looks almost black.

The following two pictures show dilutions of the dark liquid, as shown above. These two pictures show that MnO42- has a very nice bluish green color.


![]()
Oxidation state +7
Finally, the +7 oxidation state of manganese is quite well known in the common compound potassium permanganate. In aqueous solution, this forms the intensely colored purple ion MnO4–. Even dilutions of 1 : 1000000 still have a visible pink/purple color. When the concentration of a solution of a permanganate increases, then at a certain point, it looks as if the color shifts towards a bluish purple. Even at moderate concentration (e.g. 1% by weight), the liquid becomes almost black, unless a very thin layer of liquid is viewed. The following two pictures show a solution of potassium permanganate, the left one diluted to approximately 1 : 10000, the right one a few times more concentrated.


Manganese in the +7 oxidation state also can be green. When potassium permanganate is added to concentrated sulphuric acid, then manganese heptoxide, Mn2O7, is formed. A solution of this in sulphuric acid is green.


This compound, however, only has limited solubility in sulphuric acid. Oil-like droplets separate from the liquid. These droplets have a very special property. When viewed with reflected light, then they are dark green. When light is transmitted through a very thin layer of this, then it looks red. This already is shown in the picture above, but it is shown better in the right picture below.
The picture below shows such a droplet of Mn2O7, which is very dark green. The solution of Mn2O7 in sulphuric acid also is covered with smaller oily droplets, which are floating on the liquid.
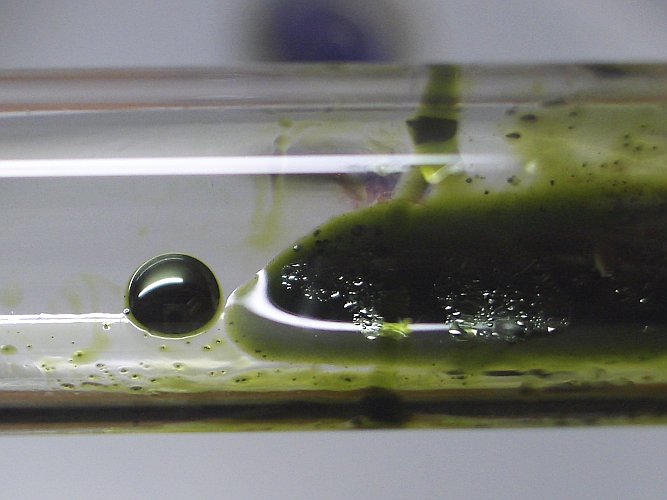
When the test tube is turned around quickly, then the oily drop breaks apart and the glass is covered by a very thin layer of the compound. Now, one can see that the compound is dark red, with light transmitted through the compound.
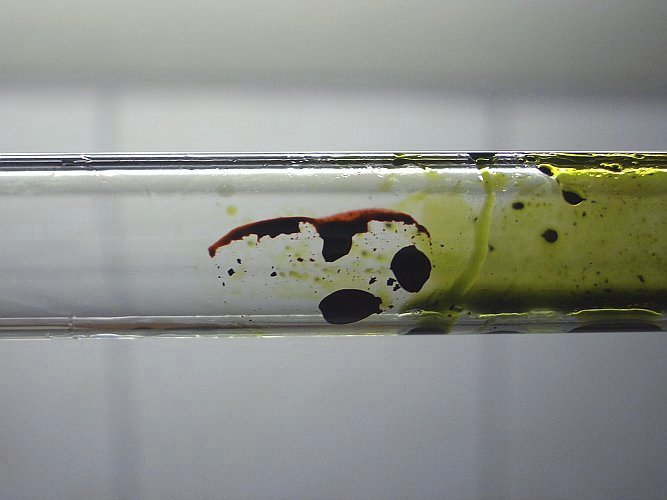
Manganese heptoxide is a very unstable compound and reacts explosively with reducing agents. Isolation of this compound is very dangerous and should not be attempted by the home chemist. The extreme reactivity of manganese heptoxide is shown nicely by this experiment.
back to solutions/precipitates main page
back to miscellaneous main page