


Miniature explosions in a test tube
In this experiment ethanol is oxidized by manganese (VII) oxide in a strongly acidic environment. This oxidation is extremely exothermic and is accompanied by small explosions, with emission of bright flashes of light.
![]() This is an impressive, but also somewhat dangerous experiment. Be very careful,
when performing this experiment!
This is an impressive, but also somewhat dangerous experiment. Be very careful,
when performing this experiment!
![]()
![]() Required
chemicals:
Required
chemicals:
-
concentrated sulphuric acid (92 - 98%, drain cleaner grade is suitable, as long as it is not too dirty)
- ethanol 96% (denatured alcohol is suitable as well)
- potassium permanganate
![]() Required
equipment:
Required
equipment:
-
test tube
- a means to fix the test tube
![]() Safety:
Safety:


- Concentrated sulphuric acid is extremely corrosive and causes severe burns when in contact with the skin.
- Ethanol is flammable.
- Potassium permanganate is a strong oxidizer.
-
 The combination of
potassium permanganate and concentrated sulphuric acid is extremely reactive and reacts
explosively with any organic compound!
The combination of
potassium permanganate and concentrated sulphuric acid is extremely reactive and reacts
explosively with any organic compound!
![]() Disposal:
Disposal:
-

 Disposal of the
material requires a special procedure for neutralization. If this procedure
is not followed carefully, then serious injury may result! The procedure for
disposal is given below in the description of the experiment.
Disposal of the
material requires a special procedure for neutralization. If this procedure
is not followed carefully, then serious injury may result! The procedure for
disposal is given below in the description of the experiment. -
The amount of potassium permanganate used in this experiment is so small, that after neutralization the waste can be flushed down the drain without severe effects for the environment.
![]()
Procedure for performing the experiment
![]() Put a
few ml of concentrated sulphuric acid in a test tube and then very carefully,
while keeping the test tube at an angle of approximately 45°, add some ethanol or denatured alcohol. This must be done, such that the ethanol
is not mixed with the sulphuric acid.
Put a
few ml of concentrated sulphuric acid in a test tube and then very carefully,
while keeping the test tube at an angle of approximately 45°, add some ethanol or denatured alcohol. This must be done, such that the ethanol
is not mixed with the sulphuric acid.
When ethanol and sulphuric acid are mixed, then a lot of heat is produced. The amount of heat is so large, that on addition of the alcohol some boiling can be observed.
After this step, the test tube contains a small amount of concentrated sulphuric acid with a layer of ethanol above it. The border between the two layers will not be sharp. The result is shown in the picture below.
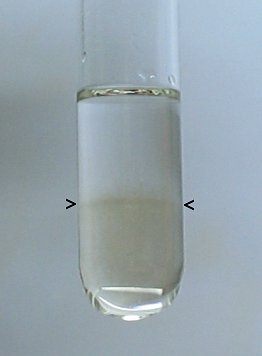
Between the two black arrows, the border between the lower sulphuric acid layer and the upper ethanol layer can be seen vaguely. It is really important to have the two layers, otherwise the experiment will not succeed. The sulphuric acid is not entirely colorless. A low-grade cheap technical quality (drain cleaner) was used for this experiment, and that is good enough.
It is also important to have a sufficiently thick layer of ethanol. If the layer is too thin, then a small explosion may result close to the surface of the ethanol and that may result in ignition of the ethanol/air mixture in the test tube. The explosions really must be inside the liquid, such that the ethanol cannot be set on fire. Inside the liquid there is no oxygen to start and support combustion of the ethanol.
![]() Once you have the two layers, fix the test tube, such that it
cannot be dropped and does not easily move. The test tube should be positioned
vertically, such that the permanganate can easily be dropped into the test tube.
Once you have the two layers, fix the test tube, such that it
cannot be dropped and does not easily move. The test tube should be positioned
vertically, such that the permanganate can easily be dropped into the test tube.
![]() Next,
add a tiny amount of solid potassium permanganate. It is sufficient to
add approximately 20 mm3. Adding more may make the reaction too
violent and may result in fire.
Next,
add a tiny amount of solid potassium permanganate. It is sufficient to
add approximately 20 mm3. Adding more may make the reaction too
violent and may result in fire.
The permanganate quickly falls through the ethanol and when it enters the sulphuric acid layer, a brown/purple compound is formed and a gas is evolved. The piece of potassium permanganate does not fall to the bottom, the bubbles of gas pull it upwards again, and it remains at the boundary of sulphuric acid and ethanol. The following two pictures show the piece of potassium permanganate falling in the acid and the subsequent formation of gas, and the accompanied rising of the piece of potassium permanganate:


The piece of potassium permanganate may remain at this position for a while, but at a certain point in time, a violent reaction will set in, with a lot of bubbling and a roaring noise. After that, it sinks to the bottom. The following two pictures show this reaction.


The following video shows the initial reaction and the roaring noise of the potassium permanganate: video of initial reaction. File size is appr. 1.5 Mbyte. Download speed depends on your internet connection. If the file does not play directly, then try to download it to your harddisk first and play the video from your local harddisk.
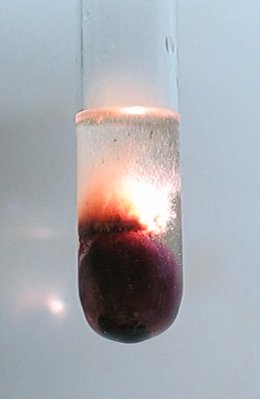
![]() After the initial violent reaction it seems that the reaction
is over, but from this point, a dark violet and brown compound is formed, which
slowly spreads across the acid. Also, the piece of potassium permanganate still
produces bubbles, albeit more slowly. After a few minutes, the acid layer
becomes filled with a dark compound completely and then suddenly, crackling
noises appear and the first flashes of light can be observed. From this point,
the mix remains crackling and flashing for many minutes and at times, there are
flashes every second. This is quite impressive, but also somewhat scary. Some
cracks are quite loud. The two pictures show the filling with the dark compound,
and one of the first, quite strong flashes.
After the initial violent reaction it seems that the reaction
is over, but from this point, a dark violet and brown compound is formed, which
slowly spreads across the acid. Also, the piece of potassium permanganate still
produces bubbles, albeit more slowly. After a few minutes, the acid layer
becomes filled with a dark compound completely and then suddenly, crackling
noises appear and the first flashes of light can be observed. From this point,
the mix remains crackling and flashing for many minutes and at times, there are
flashes every second. This is quite impressive, but also somewhat scary. Some
cracks are quite loud. The two pictures show the filling with the dark compound,
and one of the first, quite strong flashes.

The next two pictures show two other flashes. Some of the flashes are quite high up in the ethanol layer, but the bigger flashes are close to the border between the acid layer and the ethanol layer.


During the experiment, the light was switched off for a while. In a dimly lit room, the experiment is even more impressive. A larger flash inside the test tube gives a result, resembling deep-space stellar nebulae with starlight shining on them. All the white specks are due to gas bubbles, which are lit by the flash:

The following video shows the flashing and crackling noise. It nicely shows that this reaction is quite impressive and can be even somewhat scary at times: video of flashing and crackling. Beware, this is a large file, appr. 4 MByte, so it may take a while before it is downloaded.
Remark: If after a few minutes, there still are no flashes, then one can add another small amount of potassium permanganate. Some smaller crystals then are most suitable. They will get stuck in the dark compound and then quickly the flashing and crackling will start, but in many cases, the flashing and crackling will start without the need to add more potassium permanganate.
![]()
Disposal of the contents of the test tube
Disposal of the reaction mixture must be done in a special way. If the contents simply is poured out of the test tube, then there is a big chance that the ethanol catches fire, because of mixing of unused potassium permanganate and sulphuric acid, which comes in contact with ethanol and air.
The test tube must be kept at an angle of approximately 45° and it must be immersed at once in a bucket full of water. In this way, the water will quickly be mixed with the ethanol and sulphuric acid and the dangerous mixture is quenched. The contents of the test tube now can be rinsed away safely. If brown stains remain on the inside of the test tube after rinsing, then these can be removed with a small amount of acidified dilute hydrogen peroxide or a small amount of a sulfite, to which some dilute sulphuric acid is added.
![]()
Discussion of the results
Potassium permanganate reacts with concentrated sulphuric acid as follows:
2KMnO4 + 2H2SO4 → Mn2O7 + H2O + 2KHSO4
The compound Mn2O7 is an extremely reactive compound, which is very unstable. It decomposes easily, forming oxygen (bubbling), some ozone and manganese dioxide (MnO2), which can be observed as the brown matter in the pictures above. Pure Mn2O7 is an oily volatile brown/purple liquid, having a green appearance in reflected light. In the reaction mixture it can be observed as a brown/purple mess, mixed with brown MnO2 from its decomposition. As soon as some Mn2O7 comes into contact with the ethanol, a violent explosive reaction occurs, in which the ethanol is oxidized, probably to carbon dioxide and water.
The reaction between Mn2O7 and ethanol is very energetic. The temperature locally (in a bubble with a diameter in the order of magnitude of 100 μm) rises to high values, probably far beyond a thousand degrees centigrade. This explains the flashes of light and the crackling noise.