


Results of a second electrolysis experiment
This is the experimental result of a second experiment. Now, 37 grams of salts were dissolved in 150 ml of water. The remaining setup was similar, but a more accurate ampere meter was used.
Below follow some measured results in the second experiment:
| Voltage across cell (volts) | Current through cell (amperes) |
| 3.14 | 0.0739 |
| 3.19 | 0.0896 |
| 3.25 | 0.114 |
| 3.37 | 0.156 |
| 3.65 | 0.30 |
| 3.91 | 0.43 |
| 4.00 | 0.43 |
| 4.09 | 0.48 |
| 4.20 | 0.55 |
| 4.41 | 0.64 |
| 4.80 | 0.90 |
| 5.13 | 1.08 |
| 5.63 | 1.45 |
| 6.01 | 1.70 |
| 7.66 | 3.04 |
| 7.81 | 3.28 |
When these results are put in a graph, then the result is as follows:
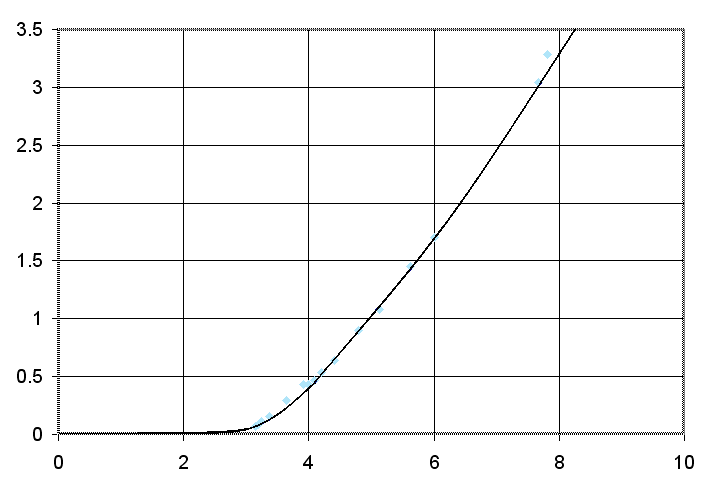
The vertical axis is the current in ampere, the horizontal axis is the voltage across the cell in volts. The light blue dots are the measurement points and a smooth curve is drawn through these points.
When these results are compared to the first experiment, then one sees that the results are matching quite well for voltages up to 5 volts. At higher voltages, however, the current is lower than in the previous experiment. It seems that there is a stronger resistive effect in this cell, than in the previous experiment. This might have been due to a not-so-good contact between one of the electrodes and the clamp, holding it, which introduces a few tenth's of ohms of resistance.
Also, obtaining really accurate measurement results is remarkably difficult. A major problem is that the rods are always covered by quite a lot of bubbles. These bubbles stick to the rods, effectively reducing the contact area. As soon as such a bubble moves upwards, and leaves the rod, the contact area is increased again and the current increases at once. This effect makes the current measurement quite noisy (the last digit of the ampere meter was changing values every second or so).
Based on these measurements, the total cell resistance is found to be somewhere between 1.2 and 1.3 Ω. The sum of both electrode overpotentials is quite high as well. For the larger currents (1 A and up) it is nearly constant around 1.4 V. This voltage is computed as the applied voltage, minus the redox potential of 2.19 V for both electrode reactions, minus the loss of voltage, due to the resistive effect of 1.2 .. 1.3 Ω.
Although at detail level the characteristics of this cell differ from the cells of the previous experiment, the general behavior is very similar again. The measured curve nicely fits a model with two zener diodes, two normal diodes in forward direction and a resistor. For normal NaCl-solution, with plain graphite rods, a voltage of approximately 5 V is a very reasonable operating voltage.
back to experiment description