


Preparation and reactions of Ba(BrO3)2 of CsBrO3
Barium bromate is an interesting and very easy to prepare chemical. It has very low solubility in cold water, while being quite soluble in hot water. This makes preparation of this chemical particularly easy. Any soluble barium salt and soluble bromate salt can be used as a starting point, but the use of sodium bromate is not recommended. This experiment shows the beautiful color of the light, emitted by a burning barium bromate / sulphur mix. Any traces of sodium ions will spoil this experiment, due to very strong yellow/orange light emission of sodium ions.
 Be
careful with the chemicals, used in this experiment. Barium-salts are very toxic
and bromates also are quite toxic. Bromate ion is a suspected carcinogen.
Be
careful with the chemicals, used in this experiment. Barium-salts are very toxic
and bromates also are quite toxic. Bromate ion is a suspected carcinogen.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium bromate *
-
any soluble barium salt **
-
sulphur, powdered
-
any soluble cesium salt (optional, only needed for second experiment with blue flames)
![]() Required
equipment:
Required
equipment:
- petri dish
![]() Safety:
Safety:
- Barium salts are very toxic. Assure that no dust or small particles of the barium salts are inhaled. Also, the smoke of the green fire, produced in this experiment, is very toxic. It contains quite some barium.
- Potassium bromate is quite toxic and a possible carcinogen. Be careful not to be exposed to this. Although potassium bromate was used in the past in baking products, better be safe than sorry. The use of potassium bromate as flour enhancer has been abandoned after evidence of potassium bromate being a carcinogen.
- The fire, produced in this reaction is quite violent. Only use small quantities.
-
 Mixing of barium
bromate and sulphur must be done with great care. Excessive friction in the
mix may cause unexpected and premature setting off of the mixture.
Mixing of barium
bromate and sulphur must be done with great care. Excessive friction in the
mix may cause unexpected and premature setting off of the mixture. -
 Do not store mixes
of barium bromate and sulphur (or any other reductor). The long-term
stability of such mixtures is not investigated and there may be a risk of
self-ignition on storage for a longer time.
Do not store mixes
of barium bromate and sulphur (or any other reductor). The long-term
stability of such mixtures is not investigated and there may be a risk of
self-ignition on storage for a longer time.
![]() Disposal:
Disposal:
- Both solid waste and solutions of barium salts must be treated as toxic chemical waste. Any barium waste, remaining after the experiment, must be brought to a proper waste processing facility.
- Remains of unused potassium bromate and solutions of potassium bromate can be flushed down the drain with a lot of water, provided that there is no barium contamination in the waste.
- Remains of cesium salts also can be flushed down the drain with a lot of water.
* Potassium bromate can fairly easily be prepared from potassium bromide. Click here for more information. For this experiment, there is no need to purify the potassium bromate. Just rinse it with some distilled water. Of course, commercial samples of potassium bromate also can be used.
** A solution of barium nitrate or barium chloride is suitable, as long as it is not acidic. Acid spoils the process of making barium bromate. A good source of barium ions is potteries barium carbonate. Dissolve an excess of this barium carbonate in some dilute hydrochloric acid or nitric acid, and heat the liquid, to assure that all acid has reacted. If not all acid wants to react quickly, then neutralize any excess with a small amount of potassium carbonate or potassium hydroxide. Do not use any sodium salt. The solution of the barium salt can be used as is, no need to isolate the barium salt. Filter away any excess insoluble barium carbonate, such that a clear and (almost) neutral solution of a barium salt is obtained. Of course, one can also use a commercial preparation of barium nitrate or barium chloride and dissolve that in water. In this experiment, shown here, a home-made solution of barium nitrate was used, which was not acidic.
![]()

Preparation of barium bromate
![]() Take a
solution of barium nitrate or barium chloride (see remark ** above). This
solution must be concentrated. If the solution is dilute, then concentrate it by
boiling off some water.
Take a
solution of barium nitrate or barium chloride (see remark ** above). This
solution must be concentrated. If the solution is dilute, then concentrate it by
boiling off some water.
![]() Be careful not to breathe any aerosol of fine droplets, coming from the boiling
solution of the barium salt.
Be careful not to breathe any aerosol of fine droplets, coming from the boiling
solution of the barium salt.
![]() Dissolve
some potassium bromate in as little as possible of hot water.
Dissolve
some potassium bromate in as little as possible of hot water.
![]() Add the
solution of potassium bromate and the soluble barium salt to each other, while
still boiling hot. Let the mix of the two solutions cool down slowly. As a rule
of thumb, use equal volumes of solution of potassium bromate and solution of the
barium salt. For optimal yield one has to take a molar ratio of barium ions and
bromate ions of 1 : 2, but even a deviation with a factor 2 from this ideal
ratio still makes a good amount of the precipitate of barium bromate. This
experiment is not critical at all.
Add the
solution of potassium bromate and the soluble barium salt to each other, while
still boiling hot. Let the mix of the two solutions cool down slowly. As a rule
of thumb, use equal volumes of solution of potassium bromate and solution of the
barium salt. For optimal yield one has to take a molar ratio of barium ions and
bromate ions of 1 : 2, but even a deviation with a factor 2 from this ideal
ratio still makes a good amount of the precipitate of barium bromate. This
experiment is not critical at all.
Quickly a white precipitate will form of crystals of barium bromate. This precipitate can easily be separated from the solution. Rinse the precipitate with ice-cold water. For the experiment below, with the green flame, there is no need to refine the crystals by recrystallization. Simply rinsing and drying is good enough. For more pure crystals, a single recrystallization is required. This can be done by dissolving the rinsed crystals of barium bromate in as little as possible of boiling distilled water. For each gram of barium bromate take approximately 10 ml of water and heat the liquid. Next, slowly add more water, while keeping it near boiling, until all of the barium bromate is dissolved. Then let cool down to 10 ºC or even colder, and very pure white crystals of barium bromate will settle. The yield is more than 90%. Impurities remain in solution.
Pure barium bromate forms very nice needle-like crystals. For this experiment, some pure barium bromate was dissolved in a small amount of water, and the solution was allowed to cool down and evaporate slowly in a petri-dish in a dust-free warm place. The result is shown in the picture below. It is a picture of the crystals on the glass of a petri-dish.

A close-up of the crystals shows their structure even better. They are very nice shiny needles, some of them having a length of more than 5 mm.

![]()
Burning a mix of barium bromate and sulphur
For this experiment, a mix of barium bromate and sulphur must be prepared in a weight ratio of approximately 4 : 1. Be careful to prepare very small amounts. For this experiment approximately 100 mg of the mix was prepared, by mixing 80 mg of barium bromate and 20 mg of sulphur.
Preparing the mix must be done by crushing the crystals of barium bromate to a fine powder, and mixing this with fine powder of sulphur. Mixing was done on a piece of aluminium foil, by carefully swirling the foil with the two chemicals in it. Do not apply pressure or strong friction, when mixing these two chemicals, otherwise premature ignition may occur. The resulting mix of chemicals is light yellow.
Lighting the mix of chemicals can be done with a flame. The mix easily ignites and burns quickly with a really beautiful bright green flame. It unfortunately is hard to capture the really beautiful green color of the flame itself with a digital camera, due to overexposure of the flame. But the green illumination of the direct neighborhood of the flame gives a good indication of its beautiful green color.
Below follows a sequence of 10 images from a small video, made of this reaction. Approximately 20 mg of the mix was put on a miniature spatula and ignited in the flame of an alcohol-burner. These 10 frames cover a time-span of 330 ms.




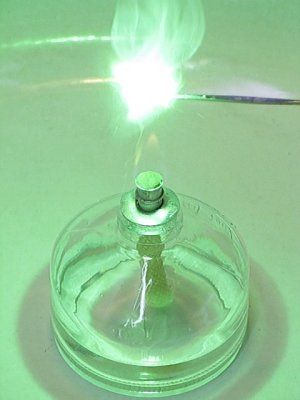





Click here for the video, from which these images were taken.
![]()
Comparison with mix of BaCO3/KBrO3/S
For comparison purposes, a mix was prepared of potteries grade barium carbonate, potassium bromate and sulphur. Approximately 75 mg of KBrO3 and 25 mg of sulphur were taken, but now approximately 50 mg of BaCO3 was added to the mix. The resulting mix also burns with a green flame. It still burns quite fast. Its color is less saturated, it is a little more bluish and more grayish.
For comparison, two images are placed next to each other, the left shows the light from the Ba(BrO3)2/S mix and the right shows the light from the KBrO3/S/BaCO3 mix. It still is a nice green, but not nearly as brilliant as the green from the Ba(BrO3)2/S mix.


![]()
Using a mix of CsBrO3/S, blue flame color
A very similar experiment also was done with cesium bromate and sulphur. This experiment is described in a separate webpage, which resembles this page on barium bromate very much.
![]()
Discussion of results
![]() The easy preparation of barium bromate from potassium bromate and a soluble
barium salt is possible, because of the low solubility of barium bromate at room
temperature, or near the freezing point of water, while the solubility of barium
bromate is reasonably high near the boiling point of water.
The easy preparation of barium bromate from potassium bromate and a soluble
barium salt is possible, because of the low solubility of barium bromate at room
temperature, or near the freezing point of water, while the solubility of barium
bromate is reasonably high near the boiling point of water.
The reaction is a simple metathesis reaction:
Ba2+(aq) + 2BrO3–(aq) → Ba(BrO3)2(s)
![]() The oxidation of the sulphur is a very exothermic
reaction. Multiple reactions occur at the same time, but the most important
reaction is the following:
The oxidation of the sulphur is a very exothermic
reaction. Multiple reactions occur at the same time, but the most important
reaction is the following:
Ba(BrO3)2 + 3S → BaBr2 + 3SO2
Side reactions also produce some free bromine, barium sulfate, and barium oxide.
During the reaction, so much heat is produced, that the reaction mix starts to emit light. The combination of barium ions and bromide ions gives rise to the deep green color. This deep green color is specific to the barium/bromide combination. If e.g. barium nitrate is mixed with sulphur, then one also obtains a very exothermic reaction with appearance of fire, but the color of the flame only is weakly green, it is more like a grayish green, while here, the color of the flame is a beautiful deeply saturated green color, which unfortunately is not easy to capture with a camera.
Remark: The mix burns very fast, probably too fast for application in pyrotechnics as a color light. Adding some compound in order to slow down the burning may also make the color even brighter. The use of carbon as reductor is not recommended, it gives an orange light when burning and most likely spoils the deep green color.