


Preparation and reactions of CsBrO3
Cesium bromate is another interesting and easy to prepare chemical. Like barium bromate, it has very low solubility in ice cold water, while being quite soluble in hot water. Preparation of this chemical is not much more difficult than preparation of barium bromate. It can easily be prepared from potassium bromate and cesium bromide by dissolving both chemicals in boiling water and letting the liquid cool down to 10 şC.
![]()
Preparation of cesium bromate
![]() Dissolve equimolar amounts of cesium bromide and potassium
bromate in as little as possible of water. Add both solids to a test tube with
some distilled water and boil, until all solid has dissolved. Taking 100 mg of
cesium bromate and 70 mg of potassium bromate is quite good. This results in
slight excess (appr. 10%) of CsBr, and that assures that less potassium is
crystallized together with the cesium bromate.
Dissolve equimolar amounts of cesium bromide and potassium
bromate in as little as possible of water. Add both solids to a test tube with
some distilled water and boil, until all solid has dissolved. Taking 100 mg of
cesium bromate and 70 mg of potassium bromate is quite good. This results in
slight excess (appr. 10%) of CsBr, and that assures that less potassium is
crystallized together with the cesium bromate.
![]() Let
liquid cool down. A white crystalline solid settles at the bottom.
Let
liquid cool down. A white crystalline solid settles at the bottom.
![]() Quickly
rinse with a small amount of ice cold water and then let the crystals dry.
Quickly
rinse with a small amount of ice cold water and then let the crystals dry.
The final result is around 100 mg of raw CsBrO3, there is no need to purify further and for this small amount, the loss of some Cs is taken for granted. A more careful preparation certainly can increase the yield somewhat. Small amounts of potassium in the final mix are no problem.
Remark: Despite the larger difference between solubilities of sodium bromate and cesium bromate, do not use sodium bromate as a starting point. Even small amounts of sodium in the final product totally spoil the experiment.
![]()
Burning a mix of cesium bromate and sulphur
For this experiment, a mix of cesium bromate and sulphur must be prepared in a weight ratio of approximately 5 : 1. Be careful to prepare very small amounts. For this experiment approximately 120 mg of the mix was prepared, by mixing 100 mg of cesium bromate and 20 mg of sulphur.
Making the mix must be done in the same way as making the mix of barium bromate and sulphur, the same safety precautions should be taken. Lighting the mix is done in the same way. The mix burns like a barium bromate/sulphur mix, albeit somewhat slower.
Below follows a sequence of 10 images from a small video, made of this reaction. Approximately 10 mg of the mix was put on a miniature spatula and ignited in the flame of an alcohol-burner. These 10 frames cover a time-span of 330 ms.



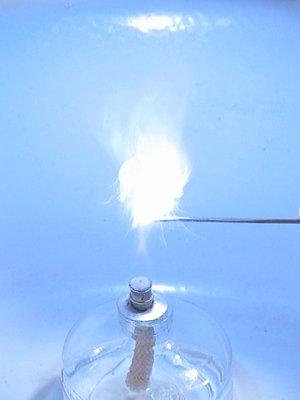




Click here for the video, from which these images were taken.
![]()
Effect of adding some anhydrous CuCl2
The cesium bromate sulphur mix already gives a beautiful blue color. It is common knowledge in the world of pyrotechnics that copper, combined with chloride can give a blue color as well. The following experiment shows the effect of combining the cesium bromate/sulphur mix with copper (II) chloride, anhydrous.
Copper (II) chloride is a brown solid, which, when finely powdered is yellow/brown. This was prepared from CuCl2.2H2O by means of careful heating. Heating too strong results in loss of HCl and formation of a basic oxychloride.
Approximately 10 mg of anhydrous copper (II) chloride was mixed with the remains of the cesium bromate/sulphur mix (estimated to be appr. ⅔ of the initially prepared mix, around 80 mg). Adding this copper (II) chloride has a striking effect on the flame color. The blue color now really is intense, and one seldomly sees such deep colors in pyrotechnic displays. But, also purplish tints can be observed, especially at the borders of the flames.
Here follow two images from burning the mix, the left without the copper (II) chloride, and the right with the copper (II) chloride. Both images have precisely the same lighting conditions and settings at the camera and both are at the brightest moment of burning. The copper (II) chloride makes the blue color much more saturated. The copper (II) chloride also makes the mix somewhat slower burning.

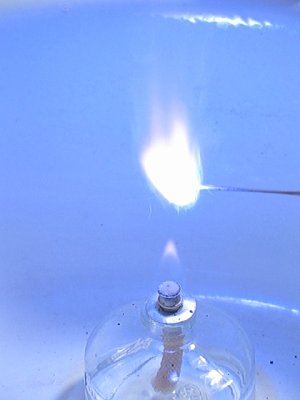
Adding the copper (II) chloride has the effect of making the mix slower burning, it makes the blue color more deep, and it results in strange purple streaks in the flame. Below follow a few pictures from a video, made of the burning cesium bromate / sulphur / copper chloride mix. Also, the flame of the alcohol burner received many beautiful colors, shades of green and blue.:


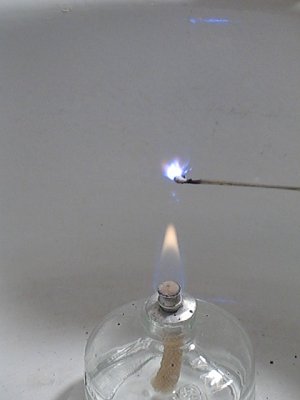
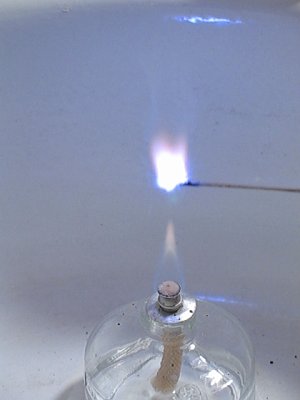




Click here for the video, from which these images were taken.
back to main page on barium bromate