


Rayleigh scattering, blue sky and red sunset
With some simple apparatus and a few very common chemicals, one can nicely demonstrate the effect, which causes the sky to appear blue on a sunny day and which causes sunset to appear red. This simple experiment is very appealing, when the right explanation is given to the audience.
Molecules and atoms can cause scattering of light, the effect being noticeable when kilometers of material are traversed by the light. Larger particles (e.g. very fine dust, or suspensions of solid particles in transparent liquids, also called colloids) also cause scattering and such scattering is noticeable even at a cm range. The effect, however, is very similar in both forms of scattering.
When the particle diameter is much smaller than the wave length of the scattered light, then blue light is scattered more than red light.
The drawing below roughly describes what happens. The white light source gives off beams of light, which are a mix of blue, red and other colors. Blue rays are scattered more than red rays. So, an observer. looking towards the particles, who does not look directly into the light source, sees light with a stronger blue content. He sees a blue haze. Another observer, also looking towards the particles, but also looking directly into the light source sees the remains of the light, which is not scattered. This light appears red, because a relatively large part of the blue light is filtered out (this is scattered in other directions).
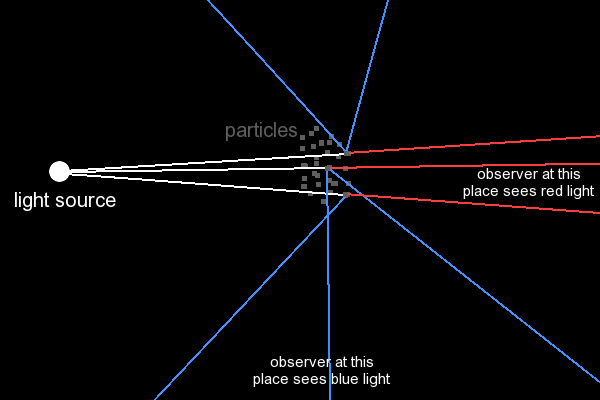
This (roughly) explains why the sky appears blue, and the sun appears red when it sets.
![]()
Required materials:
- Clear glass tank, rectangular with glass walls, volume of 2 liters
- Sodium thiosulfate (penta-hydrate or anhydrous, both are suitable)
- Dilute hydrochloric acid
- Light source, lamp
- LED's (white, UV, blue, green, yellow, orange, red)
Safety:
- Dilute hydrochloric acid is somewhat corrosive. In case of spilling, remove immediately and rinse with lots of water.
- A small amount of sulphur dioxide is formed in this experiment. The amount, released into the air, is so low, that it most likely will not cause any discomfort, but one can smell the gas.
- The light bulb, used in this experiment, can become very hot, especially when it is a tungsten bulb or halogen light. Be careful not to touch it.
![]()
Solutions, used for making a scattering colloidal liquid
In each of the experiments, 1 liter of a 1% solution of sodium thiosulfate is used. Make this solution by taking 10 grams of the salt, adding half a liter of water and dissolving this. Then add another half a liter of water and carefully stir. If anhydrous sodium thiosulfate is used, then take 6.5 grams of the solid instead of 10 grams.
For each experiment, also 300 ml of a very dilute solution of hydrochloric acid is prepared. If 30% acid is used, then take 12 ml of acid and dilute this with 300 ml of water. If 10% acid is used, then take 40 ml of acid and add water, till a volume of 300 ml is obtained.
For the solutions, plain tap water can be used, which should have a temperature of 15 to 20 �C. Be sure not to use too warm water, otherwise the reaction goes too fast. Very cold water also is not suitable. That would make the reaction too slow.
Immediately before the experiment, with the light source switched on already, add the 300 ml of dilute acid solution to 1 liter of 1% thiosulfate solution and carefully stir with a plastic spoon, in order to make the solution homogeneous. From that moment, simply watch what happens. The reaction completes in 2 to 5 minutes, depending on the temperature of the water.
![]()
Experiment 1: Scattering of light, observer who stands aside
First, an experiment is done, with light coming from above, shining through a hole in a plate, which covers a tank with a solution, which slowly becomes colloidal. The setup in this experiment is as follows:
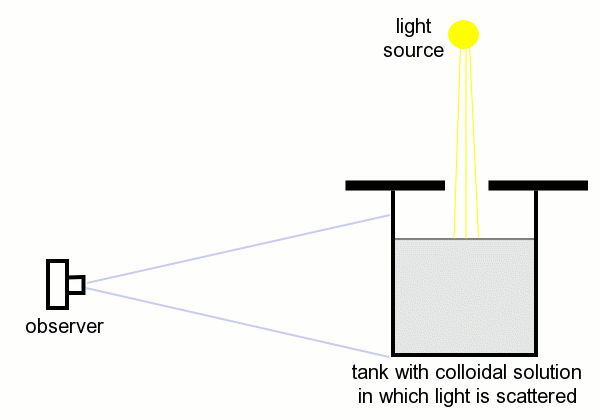
A picture of this setup, from the standpoint of the camera (observer), just before the experiment, where the solution in the tank is still clear. The acid is not yet added here.

During the experiment, the camera was zoomed in at the tank only. The solution then first remains clear for a while, but one minute after adding the acid, the liquid slowly becomes turbid and one really nicely can see the blue color of the scattered light. When the particle size in the solution increases, then the scattered light turns white. The sequence of pictures, given below shows the tank with the scattered light in intervals of 10 seconds. The first picture is 10 seconds after mixing the acid with the thiosulfate solution, the last picture is 150 seconds after mixing the two liquids.















In this particular experiment, the strongest blue color was reached after 95 seconds. A larger picture of this moment is given in the picture below. Before that, the intensity of the scattering is weaker, after that, the color of the scattered light goes towards white.

A video of the scattering effect can be downloaded. Download size of the video is 1.1 MByte.
The video runs at 5 times the real speed of the process. In reality one also can obtain a 5 times faster process, by increasing the temperature of the water. With water of 30 to 40 �C the reaction already goes much faster.
![]()
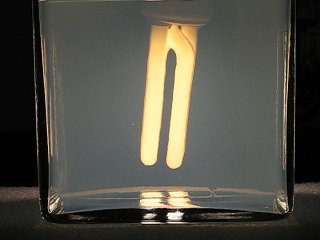
Experiment 2: Observer who looks into the light source
In the second experiment, the camera (observer) looks directly into the light source. The tank is placed between the light source and the camera.
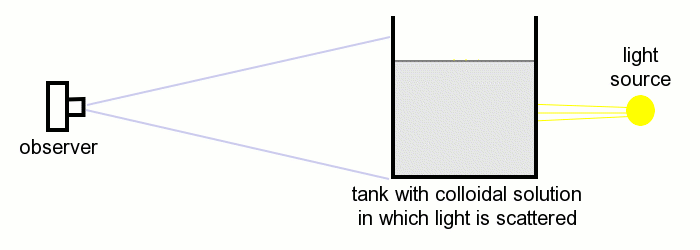
In this experiment, initially, one only sees the light source and its dark background (which almost is black). After some time, the scattering in the tank again causes the background to appear blue, but the light source itself turns yellow. When the scattering becomes stronger, then the color of the light source turns orange/red, and the light from the tank turns yellow.
Below, a set of pictures is given, at 0 seconds, 80 seconds, 90 seconds, 100 seconds, the large picture is at 120 seconds, and then 135 seconds and 150 seconds. Again, the first minute, nothing seems to happen, and then the scattering effect quickly increases.


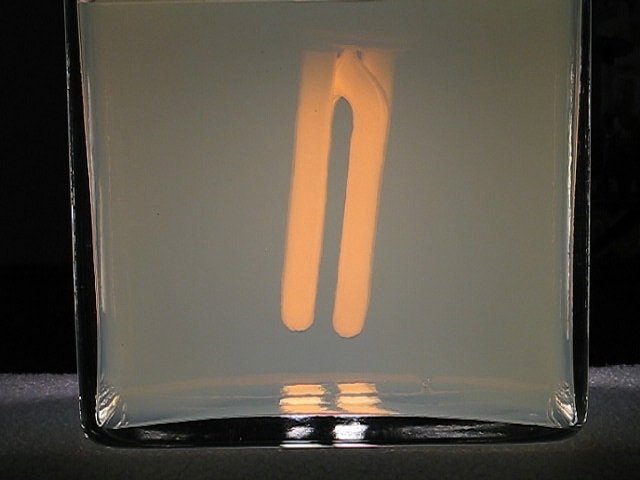


From this experiment, a video can be downloaded as well. Again, it is at 5 times the real speed. Download size is just below 1.4 MByte.
![]()
Experiment 3: Light sources with different colors
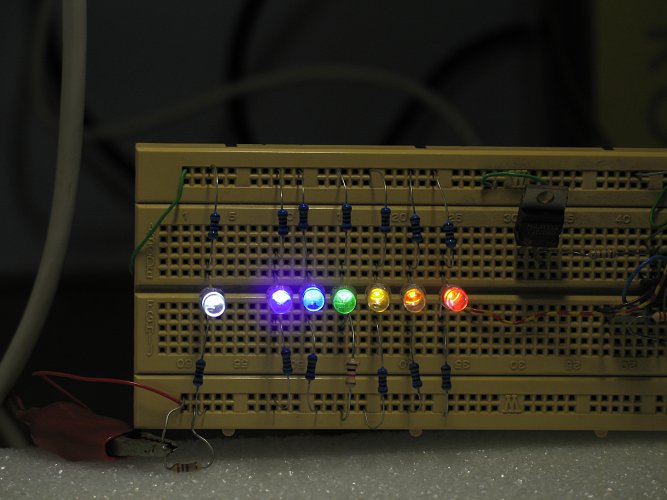
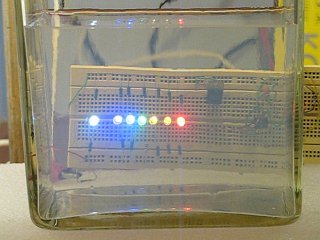
This experiment resembles the setup of experiment 2 quite a lot. The light source again is behind the tank and the observer is looking into the light source, but now, a set of LED's is used. Seven LED's were applied:
- white LED, having light from the entire spectrum
- UV (peaks at 395 nm)
- blue (peaks at 470 nm)
- green (peaks at 520 nm)
- yellow (peaks at 585 nm)
- orange (peaks at 605 nm)
- red (peaks at 625 nm)
A picture of the LED setup shows the colors. The LED's were attached to a bread-board, with a current of approximately 4 mA going through them, except for the UV-LED which has a much lower efficiency, for this LED a current of approximately 20 mA is selected. The picture below also reveals that the camera, used for making this picture is bad at handling the UV-light. All LED's look nice and sharp in the picture, but the UV-LED has a bad haze around the LED bulb. With the eye, this haze is not observed, it is an optical artefact from the camera. The UV-LED only produces a dim violet visible light, the UV-output is not visible to the eye.
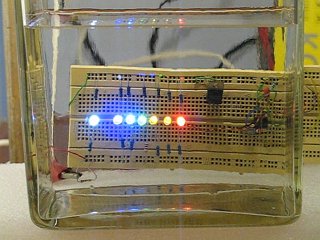
For the experiment, the following setup is used. The light bulb above the tank is put there, in order to have a decent background light and helping the camera reaching acceptable aperture settings without introducing strong overexposure. A paper bag is put over the tank, in order to avoid direct light from the light bulb shining into the liquid and disturbing the scattering effect for the LED's.

The pictures below show the result of this experiment, immediately after mixing the acid with the thiosulfate, 80 seconds after mixing, the large picture is 120 seconds after mixing, and finally 160 seconds and 200 seconds after mixing. The pictures beautifully demonstrate that red light is scattered less than blue light. The red LED remains visible for the longest time. Also, the white LED appears more and more yellow/orange until it finally fades.
The last picture only shows the red LED and when one is looking very carefully, the yellow and orange LED also can be spotted faintly.



Again, a video can be downloaded of this experiment. This video also beautifully shows that the light of the red LED can be observed for the longest time. This video also is playing at 5 times the real time speed. Download size is approximately 2 MByte.
![]()
Discussion of results
In this discussion, first the chemistry behind the reaction is explained, and next, the physics behind the scattering is explained in more depth. Especially the latter is rather involved and a quantitative understanding of this effect requires quite some mathematics. Only a very basic explanation is given. More information on this can be found in modern books on physics and optics.
Formation of colloidal solution -- chemistry behind particle formation
The chemistry behind this experiment is fairly simple. Thiosulfate ion is in equilibrium with acid, leading to an unstable entity:
2H+ + S2O32- ↔ H2S2O3
The molecule H2S2O3 is unstable and slowly decomposes:
H2S2O3 ↔ H2O + SO2 + S
The sulphur atoms slowly stick together, forming larger particles and this removes them from the equilibrium system, causing the reaction to complete. After a minute or so, these particles grow so large, that the scattering becomes visible, even when only a few cm of liquid are traversed by the light. After 5 minutes, these particles are so large, that their size exceeds the order of magnitude of the wave length of the light. Under those conditions, the liquid becomes totally opaque and the scattering does not depend on wave length anymore.
Scattering by very small particles
When the particle size is much smaller than the wavelength of the scattered light, then the intensity of observed scattered light can be expressed by the following formula:
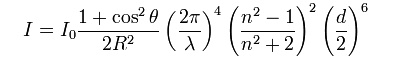
Here, I0 is the intensity of light, falling on a particle, d is the diameter of the particle, n is the refractive index of the particle, λ is the wavelength, R is the distance of the observer from the particle and θ is the angle between the original direction of the light beam and the direction of the scattered light. So, in the direction of the light beam, and in the opposite direction, the scattering is twice as intense as in directions, perpendicular to the direction of the original light beam.
Most important two features of this expression, however, are
- For decreasing particle size the scattering intensity decreases very strongly. When particle size is halved, then the scattering intensity is reduced by a factor 64. This explains why scattering, due to air molecules only is visible by looking through kilometers of air, while scattering in colloidal solutions already can be observed at a cm range.
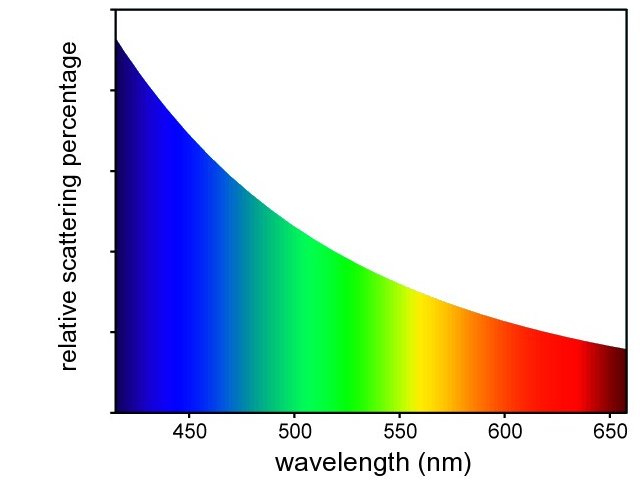
- Scattering strongly depends on the wave length of the incoming light. For the visible light, it means that at the blue/violet end of the spectrum (400 nm) the scattering is much stronger than at the other end of the spectrum (red, 650 nm). The factor is (650/400)4, which is approximately 7. So, for white light input, the scattered light is stronger near the blue end of the spectrum. The following diagram shows a qualitative picture of the the scattering as function of wave length:
Scattering by larger particles
When the particle size becomes at the same order of magnitude as the wave length, or even larger, then the formula, given above, does not hold anymore. For particles, much larger than the wave length of scattered light, the scattering does not depend on the wave length. E.g. particles of 10 μm diameter (which is appr. 20 times the wave length of visible light) scatter red light equally well as blue light. For a white light source, such scattered light also appears white.
When the particle size is at the same order of magnitude as the wave length of the scattered light, then the situation is somewhat between the two extremes, described above. The mathematical expression for that situation is very complex though. A scattering theory, valid for all ratios of particle diameter and wave length is available under the name Lorenz-Mie-Debye theory. It is based on solution of the electromagnetic wave Maxwell equations for spherical particles of arbitrary size.
Links to external sources of information
A lot of information on the subject of scattering is available online. Some good links are the following:
- http://hyperphysics.phy-astr.gsu.edu/hbase/atmos/blusky.html
- http://math.ucr.edu/home/baez/physics/General/BlueSky/blue_sky.html