

Violent decomposition of hydrazine perchlorate
In this experiment, the perchlorate salt of hydrazine is made and allowed to crystallize and this salt is decomposed by heating it. This reaction is violent and spectacular, but also very beautiful. The experiment itself is very simple, provided you have the right chemicals.



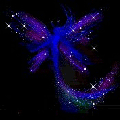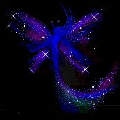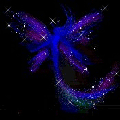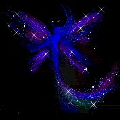
Hydrazine perchlorate is quite stable on its own. It can be kept around without problems and it is stable. On heating, however, it quickly decomposes violently with a crackling noise and beautiful effects like spraying around of glowing fairy powder.
![]()
![]() Required
chemicals:
Required
chemicals:
-
perchloric acid, 20% solution in water
-
hydrazine, 20% solution in water
![]() Required
equipment:
Required
equipment:
- test tubes
- glass rod
- pH paper (multi-range 4 ... 11)
- petri dish or hour glass
![]() Safety:
Safety:
- Perchloric acid at a concentration of 20% is moderately corrosive. Acid of much higher concentration, however, is highly corrosive and acid with a concentration of well above 70% can even cause explosions when it comes in contact with organic matter.
- Pure hydrazine is corrosive, prone to explosive decomposition and fumes in contact with air, but the 20% solution in water can be handled without any problem. Keep in mind though that hydrazine is a carcinogen and avoid exposure to its vapors.
![]() Disposal:
Disposal:
- No waste should remain, all solid material can be decomposed above a flame.
![]()
Preparation of solid ethylene diamine perchlorate
One molecule of hydrazine combines with one molecule of perchloric acid. The salt with formula N2H5ClO4 is formed. If the amounts below are used, then the synthesis certainly succeeds. Assure that no excess amount of perchloric acid remains. If excess hydrazine is present, then that simply evaporates. If excess perchloric acid is present, then a terribly hygroscopic and wet mess is obtained and no good dry crystals can be obtained, so it is best to assure a slight excess amount of hydrazine.
![]() Take 2.5 ml of a 20% solution of perchloric acid and put this
in a test tube.
Take 2.5 ml of a 20% solution of perchloric acid and put this
in a test tube.
![]() Take 1
ml of a 20% solution of hydrazine. This is a slight excess amount, relative to
the perchloric acid. Just to be sure, take a glass rod, dip this in the liquid
and transfer some adhering liquid to a piece of pH paper. This paper should
indicate a slightly alkaline solution. If the paper indicates an acidic
solution, then add a few more drops of solution of hydrazine.
Take 1
ml of a 20% solution of hydrazine. This is a slight excess amount, relative to
the perchloric acid. Just to be sure, take a glass rod, dip this in the liquid
and transfer some adhering liquid to a piece of pH paper. This paper should
indicate a slightly alkaline solution. If the paper indicates an acidic
solution, then add a few more drops of solution of hydrazine.
![]() The
resulting liquid (which has a slight excess of hydrazine) is transferred
to a hour glass or petri dish and the glass or dish is put in a warm dry place
(e.g. on a central heating radiator). Let the liquid evaporate for one day.
After one day, a crystalline layer is formed in the petri dish. Scrap the solid
from the glass and spread out the crystalline material again. Allow drying for
another day. After this period, perfectly dry and non-hygroscopic crystals are
obtained. The picture below shows the crystals, when added to a big test tube
and photographed from above through the test tube.
The
resulting liquid (which has a slight excess of hydrazine) is transferred
to a hour glass or petri dish and the glass or dish is put in a warm dry place
(e.g. on a central heating radiator). Let the liquid evaporate for one day.
After one day, a crystalline layer is formed in the petri dish. Scrap the solid
from the glass and spread out the crystalline material again. Allow drying for
another day. After this period, perfectly dry and non-hygroscopic crystals are
obtained. The picture below shows the crystals, when added to a big test tube
and photographed from above through the test tube.

The crystals look white in the picture, but in reality they are glass-like and transparent and perfectly colorless. A close-up of the crystals shows more details:

![]()
Igniting the solid material above a flame
The solid material can be put on a small spatula and kept above a flame. The solid first melts and sputters, giving rise to formation of beautiful fairy-like "glowing powder" and then suddenly the entire mass sets off with production of lots of glowing gas. No smoke remains. The reaction is not really explosive, but it goes fast and is quite spectacular.




A video of the reaction can be downloaded here. Download size is approximately 3 MByte.
![]()
Discussion of results
![]() Hydrazine can be considered a diamine with two amino groups directly connected
to each other. Both groups can accept a proton, but the second group does so
with much more difficulty than the first group. This is because of the direct
connection between the two -NH2 groups. In this experiment, only one
group is protonated:
Hydrazine can be considered a diamine with two amino groups directly connected
to each other. Both groups can accept a proton, but the second group does so
with much more difficulty than the first group. This is because of the direct
connection between the two -NH2 groups. In this experiment, only one
group is protonated:
H2NNH2 + H+ → H2NNH3+
This ion easily forms salts with suitable anions. Commercial salts of this ion are available, such as the chloride and the sulfate.
![]() With
perchloric acid, the corresponding salt H2NNH3ClO4 is
formed. This salt forms colorless crystals which are not hygroscopic and which
easily can be prepared by evaporating a solution of this.
With
perchloric acid, the corresponding salt H2NNH3ClO4 is
formed. This salt forms colorless crystals which are not hygroscopic and which
easily can be prepared by evaporating a solution of this.
![]() The
fairy powder effect is obtained due to boiling. A drop of the molten material is
hanging under the spatula and this starts boiling. Little droplets of this
boiling material decompose, giving bluish light and producing a noise which is
somewhat between crackling and hissing.
The
fairy powder effect is obtained due to boiling. A drop of the molten material is
hanging under the spatula and this starts boiling. Little droplets of this
boiling material decompose, giving bluish light and producing a noise which is
somewhat between crackling and hissing.
![]() The
solid salt contains both the fuel and the oxidizer for the combustion. There is
excess oxygen in the compound. All reaction products are gaseous and that
explains the large fire ball and the lack of smoke. The orange color really is
due to the glowing of the gas itself, it is not due to glowing particles like in
a normal fire. For this reason, the orange glow is more 'ghost' like and no
sharp flames are produced.
The
solid salt contains both the fuel and the oxidizer for the combustion. There is
excess oxygen in the compound. All reaction products are gaseous and that
explains the large fire ball and the lack of smoke. The orange color really is
due to the glowing of the gas itself, it is not due to glowing particles like in
a normal fire. For this reason, the orange glow is more 'ghost' like and no
sharp flames are produced.
H2NNH3ClO4 → O2 + HCl + 2H2O + N2
The reaction products are gaseous and colorless, hence no visible smoke nor visible gas can be perceived.