


Dendritic crystal structures
Some chemicals have interesting properties, when they crystallize from aqueous solution. A nice example of such a chemical is ammonium chromate, (NH4)2CrO4.
Ammonium chromate is a golden yellow compound. Its color differs quite a lot from the color of potassium chromate, which is very bright yellow . Ammonium chromate is more golden yellow .
On the other hand, both potassium dichromate and ammonium dichromate are bright orange .
A solution of ammonium chromate in water is not purely yellow, it is somewhat orange/yellow. This is due to the slightly acidic properties of the ammonium ion. The following equilibrium will be present in aqueous solution:
2NH4+ + 2CrO42- ↔ 2NH3 + H2O + Cr2O72-
This equilibrium makes the liquid look orange/yellow instead of pure yellow. In solution, dichromate ion also is bright orange, chromate ion is bright yellow. Such a solution of ammonium chromate in water has a faint smell of ammonia.
When a concentrated solution of ammonium chromate is allowed to evaporate to dryness, then nice dendritic crystalline structures are formed, but at the same time, one also can nicely see the formation of both ammonium dichromate and the formation of ammonium chromate.
![]()
![]() Required
chemicals:
Required
chemicals:
-
ammonium chromate
![]() Required
equipment:
Required
equipment:
-
petri dish
![]() Safety:
Safety:
- Ammonium chromate is toxic. It contains hexavalent chromium and there is strong evidence that hexavalent chromium is a carcinogen. Be sure not to be exposed to this. Especially the route of inhalation is a risky route. Ammonium chromate is non-hygroscopic and a dry powder. Dust is formed easily.
![]() Disposal:
Disposal:
- The waste should not be thrown away with normal household waste. One nice thing to do is scrape off all crystalline solid and decompose it by heating. This reaction is well known (ammonium dichromate volcano reaction). The solid chromium (III) oxide then can be wrapped in some paper and put in normal household waste.
Remark: If no ammonium chromate is available, then this experiment also can be done by dissolving some ammonium dichromate in household ammonia, such that a golden yellow solution is obtained, which only has a faint smell of ammonia. Such a solution can be considered as a solution of ammonium chromate in slight excess of ammonia.
![]()
Formation of crystals of ammonium (di)chromate
Dissolve a spatula full of ammonium chromate in a few ml of water and then pour this solution in a petri dish. The petri dish should be placed in a dry and warm place, and it should be somewhat tilted, such that the solution is at one side of the petri dish.
Let the solution evaporate. This may take between several hours and a few days, depending on temperature and humidity of the air. When all water has evaporated, then a beautiful crystalline structure remains.
There also is a remarkable effect, that the crystals, which are oldest (are dry first) are orange, while the newest crystals are yellow. If one waits too long, then all crystals become orange, so it is important to monitor the petri dish and inspect it every few hours. Ammonium chromate is not air-stable. In contact with air, it slowly looses ammonia and water, leaving ammonium dichromate behind.
Immediately after evaporation of the water, it looks as follows:

This very nicely demonstrates the beautiful orange color and the yellow color. Also, the type of crystals changes. Initially, the crystals are dendritic, later on, the crystals are more needle-like. The picture below shows the same petri dish, but with a white paper background. Now the difference between the yellow chromate and the orange dichromate is even more pronounced.

![]()
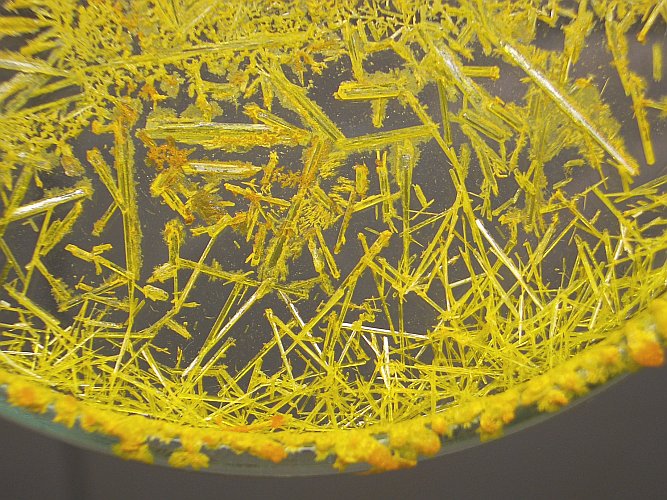
Close ups of crystal structures
The first picture is shown again, with two rectangles, drawn in it. These rectangles show where the close ups are taken.

The first close up shows the orange ammonium dichromate dendritic structures. The second close up shows the needle-like yellow ammonium chromate.

![]()
Discussion of the results
As mentioned already, a solution of ammonium chromate in water in reality contains both chromate and dichromate ions and it also contains free ammonia, due to an equilibrium reaction between the somewhat basic chromate ion and the somewhat acidic ammonium ions.
The solution slowly looses ammonia, and solid ammonium chromate also slowly looses ammonia (and water). The crystals, which appear first, are orange already, and very fine feather-like structures also become orange, quickly after they are formed.
Ammonium dichromate forms beautiful dendritic crystals, when it is allowed to crystallize from a thin layer of solution. Ammonium chromate forms needle-like crystals. It has another chemical composition and hence it has another crystal lattice.