


Conversion of phosphorus, red to white
This webpage describes how red phosphorus can be converted to white phosphorus fairly easily. Red phosphorus, although not available everywhere, still can be purchased in many countries, while white phosphorus is much harder to find. For that reason, it may be interesting to convert some red phosphorus into white phosphorus. The purity of the white phosphorus, obtained in this way is fairly good, although it certainly cannot reach the quality of commercial samples. The material, obtained in this way, however, is perfectly suitable for all kinds of spectacular experiment with white phosphorus.


 This
experiment is one of the most risky experiments from the chemistry section
of this website, due to the large risk of fire. White phosphorus is EXTREMELY flammable and very poisonous. It
also is corrosive and can give rise to nasty wounds, which are only healing very
slowly. This experiment MUST be conducted outside, with nothing flammable and
valuable nearby, or in a very good fume hood, with glass protection wall. Some
vapor of white phosphorus may be emitted. These vapors are intensely poisonous.
That is another reason why this experiment must be conducted outside.
This
experiment is one of the most risky experiments from the chemistry section
of this website, due to the large risk of fire. White phosphorus is EXTREMELY flammable and very poisonous. It
also is corrosive and can give rise to nasty wounds, which are only healing very
slowly. This experiment MUST be conducted outside, with nothing flammable and
valuable nearby, or in a very good fume hood, with glass protection wall. Some
vapor of white phosphorus may be emitted. These vapors are intensely poisonous.
That is another reason why this experiment must be conducted outside.
The main principle behind this experiment is that red phosphorus, when heated strongly, decomposes and sublimes in the form of P4 molecules. The P4 can be condensed on a cooler place to white phosphorus. A special distillation setup, capable of handling very high temperatures, combined with the use of an inert atmosphere, would be required to perform such a sublimation of red phosphorus. On a small scale, it can be done with a simple test tube, which is prepared in a special way.

![]()
![]() Required
chemicals:
Required
chemicals:
-
red phosphorus
-
marble chips (calcium carbonate)
-
hydrochloric acid (needed for "safe" cleanup)
-
bleach or calcium hypochlorite (needed for "safe" cleanup)
![]() Required
equipment:
Required
equipment:
-
test tube, made of Duran« glass
-
hot propane torch
-
clamp for test tube
-
glass wool
![]() Safety:
Safety:
-
 See warnings
above, this experiment MUST be conducted outside!
See warnings
above, this experiment MUST be conducted outside!
![]() Disposal:
Disposal:
-
 Any white phosphorus waste (e.g. on test tube walls,
in a tub, in glass wool, in tissues) must be destroyed. Nothing may remain behind, due to
the extreme risk of fire. A special section of this webpage is devoted to
the cleanup after performing the experiment. The cleanup itself also is a
rather nasty job, but the final result is a safe situation, without risk of
fire.
Any white phosphorus waste (e.g. on test tube walls,
in a tub, in glass wool, in tissues) must be destroyed. Nothing may remain behind, due to
the extreme risk of fire. A special section of this webpage is devoted to
the cleanup after performing the experiment. The cleanup itself also is a
rather nasty job, but the final result is a safe situation, without risk of
fire.
![]()
Preparation of special "distillation device"
A test tube must be bent in a special way, such that it contains the part where the red phosphorus can be heated and at the same time it contains the part where the white phosphorus can condense and can be collected.
The test tube must be one of high quality Duran« glass. Ordinary glass certainly will crack in this experiment, with possibly disastrous consequences. Duran« glass is not easily melted, but with a hot propane torch this is possible, when the test tube is kept in the tip of a narrow roaring flame. The test tube is slowly rotated and the place which must be bent is kept in the roaring flame. At a certain point, the glass starts glowing. At that point, the test tube can be bent carefully and slowly. If the bending angle is sufficiently large, then take it out of the flame and let it cool down. After cooling down, a similar thing is done with the second bend. Use plastic clamps for manipulating the test tube. Although the glass becomes amazingly hot at the place where it is bent, near the bottom and the top of the test tube it only becomes warm, one can even touch it without getting burnt. But for convenience and safety it is better to use a clamp for manipulating the test tube.
The final result is shown in the picture at the start of the
webpage. The red phosphorus is put in the bottom part of the test tube, the
![]() - shaped part of the
bent
test tube collects the condensed and liquid white phosphorus.
- shaped part of the
bent
test tube collects the condensed and liquid white phosphorus.
![]()
Filling the "distillation device" and preparing for heating
The bent test tube first must be filled with some red phosphorus, and after that, it must be flooded with carbon dioxide. The carbon dioxide can be prepared by putting some marble chips in dilute hydrochloric acid and the gas must be delivered into the bent test tube with a thin flexible tube, which is pushed into the powdered red phosphorus. Be sure to make a lot of carbon dioxide, such that really all air in the bent tube is displaced by the gas. Production of carbon dioxide must not be too fast, otherwise the red phosphorus may be blown out of the test tube.
Immediately after that, put some glass wool in the upper part of the test tube, such that the carbon dioxide does not quickly escape from the tube. Immediately after that, attach the test tube to a clamp in a position as shown in the picture below.

At the left, one can see the glass wool (most of it is covered by the clamp), at the right, one can see the red phosphorus, which will be heated.

The test tube must be loosely closed with a piece of glass wool. Do not use a rubber stopper or a tightly fitting cork. Such a stopper might pop out of the test tube during heating (pressure buildup, due to expanding gas) and that will result in instant fire.
![]()
Heating the red phosphorus, making white phosphorus
With the setup as described in the section above, the experiment can be performed relatively safely. One does not have to fear ignition of the phosphorus inside the test tube. The only real risk is cracking of the test tube, while the red phosphorus is heated. If that occurs, then there will be instant violent fire and burning drops of phosphorus will be sprayed around. Be prepared for such an event, and assure that you can quickly step away if this occurs. Also wear adequate eye protection and leather gloves during the heating part of this experiment. If the test tube cracks, then quickly step aside and let the phosphorus burn. Do not try to extinguish the fire, this is nearly impossible. For this reason, this experiment MUST be performed outside, at a place where a phosphorus fire cannot do any harm. White phosphorus burns are particularly nasty, because burning droplets are sprayed around all over the place.
In order to reduce the chance of cracking of the test tube, first heat the entire part, where the red phosphorus is up to the bend (the right part of the test tube in the previous picture, the left part in the picture below). This heating must be done evenly. After this initial heating, the flame of the propane torch must be pointed below the red phosphorus and it must be kept at that place for a while. When this is done, one quickly sees formation of yellow/white fumes.

At a certain point, the flame of the torch must be put to
full blast, but the test tube may not become so hot that it melts. Slowly, the
red phosphorus sublimes and impure white phosphorus condenses in the cooler part
of the test tube, which is the
![]() - shaped part of the
test tube. The result is shown in the picture below.
- shaped part of the
test tube. The result is shown in the picture below.

The picture shows the remains of the red phosphorus, which is a black solid (impurity of the red phosphorus, this most likely is another allotrope of phosphorus, which is much less reactive than red phosphorus) and a liquid blob of white phosphorus, which appears red due to contamination with small particles of red phosphorus. Also, drops of white phosphorus appear on the upper side of the test tube. These can be heated a little as well, in order to have all the phosphorus collected into a single blob.
At the end of the experiment, all red phosphorus has sublimed, only some black crud remains, as shown in the picture below, which was taken from above.

The resulting white phosphorus is shown in more detail in the following picture:

When this situation is reached, then let the test tube cool down, with the glass wool still in its opening. Never remove the glass wool, as long as the test tube is not completely under water! Opening of the test tube will result in instant fire!
![]()
Working up the white phosphorus, purifying to some extent
When the test tube has cooled down, then take it out of the clamp and immerse it in a tub, full of cold water. When it is completely under water, then the glass wool can be removed and the water flows into the test tube and air/carbon dioxide bubbles to the surface of the water. When cold water is used, then the white phosphorus solidifies. Once the test tube is full of cold water, it can be taken inside, and further workup can be done inside. The picture below shows the test tube, full of cold water, with the solidified blob of white phosphorus and the glass wool also inserted again.

Further working up can be done in a tub, filled with a layer of 10 cm of warm water. Take water, which is quite warm, but not so hot that you can't stand working in it with your bare hands.
Keep the test tube still full of water (with the glass wool plugged in and the test tube kept in the same orientation as when it was heated with the propane torch!) under a tap with hot running water, e.g. while filling the tub, in which further work up is done. The phosphorus will melt again.
Next, take a small glass beaker and put this in the tub with warm water. The beaker must be completely under the warm water, and a few cm of water must be above the beaker. Keep the test tube with the molten phosphorus under water, above the beaker, and then remove the glass wool and carefully pour the white phosphorus into the beaker. All of this must be done under the warm water, otherwise instant fire will be sprayed around!
Try to avoid pouring in some of the black crud as well. For this reason, it is important that during the melting of the white phosphorus, the test tube is kept in horizontal position, such that no white phosphorus by accident flows to the bottom of the test tube and is contaminated with the black crud.
Once all phosphorus is in a beaker, the beaker can be taken out of the tub. The raw liquid material at the bottom of the beaker looks as follows:

A small video shows the material in the beaker under a layer of warm water. It is a fairly mobile liquid, which does not stick to the glass. The video can be downloaded by clicking here, its download size is approximately 2 MByte.
![]()
Further purification by filtering
The white phosphorus, as prepared according to the procedure given above, already is suitable for many experiments, but one can fairly easily purify the liquid phosphorus by means of filtering through glass wool under water.
Again, all work must be done completely under warm water. Never allow air to come in contact with the liquid white phosphorus!
The white phosphorus does not stick to glass. One can easily suck the phosphorus into a glass pipette with 5 to 10 ml volume. In order to do so, the beaker with the phosphorus must be put under warm water again, and the pipette also must be put under water, assuring that most air is out of it. Under water, first suck in some water, and then suck in all crude white phosphorus. Next, while keeping all of the material and the pipette under water, take a piece of glass wool and keep this in front of the opening of the pipette and then carefully press out the phosphorus above the beaker. If this is done, a lot of solid crud remains behind in the glass wool and the white phosphorus goes through the glass wool. Only use a thin layer of glass wool, otherwise you will have to press too strongly, with the risk of pressing all of the white phosphorus out of the pipette at once, resulting in many small globules under the water, which need to be collected again.
After this purification step, the phosphorus still is not white. It has a brown/yellow color after this purification step, but better results cannot easily be obtained with the simple equipment, used in this experiment. The yellow phosphorus, obtained in this way, however, is perfectly suitable for synthesis work, and also for all kinds of experiments, which require white phosphorus.
The beaker with the purified white phosphorus can be taken out of the tub and the warm water can be replaced by cold water. With this replacement of water, again assure, that the phosphorus does not come in contact with air.
Once the phosphorus has solidified and cooled down to 15 ║C or so (the temperature of the cold water), it can be transferred to a petri dish, filled with water. Brief contact with air now does not lead to instant fire anymore. With a sharp knife it can be cut into smaller pieces, while it is under a thin layer of water in the petri dish. After cutting, the smaller pieces have to be put in a little vial, filled with cold water. Do not take pieces of white phosphorus in your hands. The pieces may ignite and cause severe burns. Use a pair of tweezers for transferring the small pieces from the petri dish to the vial.
The final result is the following, which is approximately 3 grams of white phosphorus:

The solid is soft and waxy. A rounded glass stick can easily be pressed into the solid. A sharp knife fairly easily cuts the solid.
![]()
Cleaning up of all tools, glassware and tub
All items, which have been in contact with the white phosphorus, may have solidified droplets or globules of white phosphorus on them or in them. It is very important to thoroughly cleanup any remains of white phosphorus. Simply wiping off with a tissue and then throwing away of this tissue introduces an unacceptable risk of fire, especially on warm summer days. White phosphorus can easily ignite when in contact with air, even at room temperature.
As a demonstration of the danger, the glass wool, which was in the test tube during heating, is kept in air for a while. The picture below shows how much smoke is produced from this piece of glass wool. Knowing that this piece of glass wool is completely soaked with cold water makes it even more scary. A video also shows this smoking of the wet glass wool. Download size is appr. 1.25 MByte.

Throwing such smoking material in the trash bin, introduces a serious risk of fire. Such a fire may occur, hours later, when most of the adhering water has evaporated. All white phosphorus hence must be destroyed.
A sure method of destruction is the use of a bleach solution, to which a slight excess of hydrochloric acid is added. One can also use calcium hypochlorite (swimming pool tablets or granules), which is added to 10 times its volume of water, and then some hydrochloric acid is added until the liquid becomes clear and green/yellow. Bubbles of chlorine will be produced in the liquid, as if it were a soda drink. Immerse the glass wool in this liquid for a while. Also pour this liquid into the bent test tube. The tub also must be treated with this liquid. Any tissues, brushes or whatever is used for cleaning also must be treated with the chlorine-water. Avoid contact of the chlorine-water with the skin.
Let the chlorine-water do its work for 5 minutes and then replace it with fresh liquid. During the cleaning up, quite some white smoke may be produced from specks of white phosphorus, which are exposed to air. This is not dangerous, but it looks weird and somewhat scary.
Perform the cleaning action outside, or in a very well ventilated room. Chlorine gas is very poisonous and has an extremely choking odor. Fortunately, its warning properties are good. Even at low concentration, chlorine gas is unbearable. Do not make too much of the chlorine-water at once, use multiple small batches.
After cleaning, all materials must be rinsed with water, and then the materials which are thrown away can go in the trash bin. The cleaning fluid can be flushed down the drain and after this, a tub full of water can be used to rinse away all chlorine-water.
After one minute of standing in the acidic chlorine water, the bent test tube almost is free of white phosphorus. Only two spots of white phosphorus remain in this particular situation as shown in the picture. After three more minutes of standing in fresh chlorine water, all orange/brown spots are gone, and only the black material remains. This is not a problem, the black material is inert.

![]()
The white phosphorus in action
After all this effort it is nice to see how the white phosphorus burns. It is exceptionally easily ignited, and it burns with a heavily smoking flame. It also burns while spraying around many small droplets, due to boiling of the very hot droplet of molten phosphorus. This makes white phosphorus fires exceptionally dangerous.

The spraying around is nicely demonstrated by the following picture (which I did not make myself) of burning a much larger amount of white phosphorus.

A video also demonstrates the burning of white phosphorus. Only a very small piece of phosphorus (just a few mm│) was put on the tip of the screw driver, shown in the video, and ignited. Download size is approximately 3.5 MByte.
![]()
Discussion of results
Red phosphorus and white phosphorus are different allotropes of the same element. Red phosphorus consists of phosphorus atoms, arranged in an irregular pattern of atoms. It is an amorphous solid of irregular macromolecules. In this arrangement, the atoms of phosphorus are fairly tightly bound to the macromolecules structure and this makes the solid quite stable and inert at room temperature. It also makes red phosphorus (like carbon, silicon, and boron) insoluble in any known solvent. If red phosphorus does dissolve in some solvent, then it is part of a chemical reaction and it is converted to another compound.
White phosphorus consists of P4 molecules with a particular structure, in which there is considerable strain in the molecule.
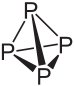
When red phosphorus is strongly heated (400 to 500 ║C) then the large macromolecular structures are broken down and atoms of phosphorus escape from the solid. These combine to molecules of P4 and at colder places, this condenses on the glass.
The strain in the P4 molecules makes them so reactive. White phosphorus can ignite at a temperature as low as 30 ║C. Red phosphorus must be heated to at least 240 ║C before it ignites.