


A volatile fluoro-manganese complex
Manganese in its +7 oxidation state can form volatile compounds with sulphuric acid and even more so, with hydrofluoric acid. Probably, a fluoro-oxo compound of manganese in its +7 oxidation state is formed. On addition of water, this complex is destroyed again and the deep purple MnO4– ion is formed.
![]() This experiment is remarkable, but also quite risky. The experiment involves
extremely corrosive, toxic and possibly explosive compounds. For this reason, it
is strongly advised not to scale up the experiment.
This experiment is remarkable, but also quite risky. The experiment involves
extremely corrosive, toxic and possibly explosive compounds. For this reason, it
is strongly advised not to scale up the experiment.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium permanganate
-
sodium fluoride
-
sulphuric acid, concentrated (95+ % H2SO4)
-
potassium metabisulfite (sodium sulfite is OK as well)
![]() Required
equipment:
Required
equipment:
-
test tube (beware, the test tube is ruined by this experiment)
![]() Safety:
Safety:
- Concentrated sulphuric acid is very corrosive to human tissues. It chars skin and many other compounds in seconds. Be really careful not to get any sulphuric acid on your skin and keep a lot of clean water nearby, just in case something happens.
- Potassium permanganate is a strong oxidizer.
- Sodium fluoride is quite toxic and fairly corrosive.
-

 The mix, created
in this experiment, is EXTREMELY corrosive and EXTREMELY toxic. Getting a
drop of this mix on your skin results in instant destruction of the skin and
formation of deep and severely painful wounds! The main constituent of the
fumes, produced in this reaction are hydrogen fluoride!
The mix, created
in this experiment, is EXTREMELY corrosive and EXTREMELY toxic. Getting a
drop of this mix on your skin results in instant destruction of the skin and
formation of deep and severely painful wounds! The main constituent of the
fumes, produced in this reaction are hydrogen fluoride! -
 The mix, created
in this experiment, also is an exceedingly strong oxidizer. Addition of any
reducible compound, like organics, results in fire or explosion. However, if
one uses the amounts, suggested below, then the risk of explosion or fire is
not too severe. One must not scale up the experiment!
The mix, created
in this experiment, also is an exceedingly strong oxidizer. Addition of any
reducible compound, like organics, results in fire or explosion. However, if
one uses the amounts, suggested below, then the risk of explosion or fire is
not too severe. One must not scale up the experiment!
![]() Disposal:
Disposal:
- After the experiment, dilute with a lot of water and carefully flush down the drain and rinse afterwards with a lot of water.
![]()
Procedure for performing the experiment
![]() Take
a small amount of potassium permanganate, less than 10 mg! This is just a
few mm3 of solid potassium permanganate. If the crystals are somewhat
large, then first crush them. Do not scale up! Put the small amount of
potassium permanganate in a dry test tube. No small crystals should stick at the
walls of the test tube. All must be at the bottom.
Take
a small amount of potassium permanganate, less than 10 mg! This is just a
few mm3 of solid potassium permanganate. If the crystals are somewhat
large, then first crush them. Do not scale up! Put the small amount of
potassium permanganate in a dry test tube. No small crystals should stick at the
walls of the test tube. All must be at the bottom.
![]() Add a spatula of solid sodium fluoride. The exact amount is
not really critical. In this experiment, a quantity of approximately 100 mg is
used.
Add a spatula of solid sodium fluoride. The exact amount is
not really critical. In this experiment, a quantity of approximately 100 mg is
used.
![]() To the mix of NaF and KMnO4 carefully add 0.2 to
0.3 ml of concentrated sulphuric acid and carefully heat to approximately 60 °C.
To the mix of NaF and KMnO4 carefully add 0.2 to
0.3 ml of concentrated sulphuric acid and carefully heat to approximately 60 °C.
On heating, the liquid starts foaming a little and the 0.2 ml
of liquid expands to approximately 1.5 to 2 ml of foam. This foam is green. The
bubbles consist of hydrogen fluoride, which also carries a volatile compound of
manganese. At the glass, the volatile compound settles again and forms a
reddish/purple layer on the glass. The pictures below show all phenomena. Above
the liquid/foam, a white fume can be observed. This is due to formation of
hydrogen fluoride, which fumes with water from the air. At the open end of the
test tube, one also can observe a white fume. This is hydrofluoric acid, formed
from hydrogen fluoride, and water vapor from the air.
![]() Beware, do not
inhale any amount of this fume.
Beware, do not
inhale any amount of this fume.



![]() Next, add a single drop of water to the test tube, while it is kept under a
certain angle. The drop of water should not be dripped directly into the green
foam. Later on, a second drop of water is added, which is allowed to drip
directly onto the green foam.
Next, add a single drop of water to the test tube, while it is kept under a
certain angle. The drop of water should not be dripped directly into the green
foam. Later on, a second drop of water is added, which is allowed to drip
directly onto the green foam.
As soon as the drop is running along the glass, the green complex and the reddish stain on the glass react to form purple permanganate. Apparently the fluoro complex of manganese is hydrolysed with the water to form permanganate and hydrofluoric acid. The glass is corroded severely at the place, where the water is running along the glass. The following pictures show what happens after adding one drop and after adding a second drop, which also runs into the green foam. The first two pictures show the situation after adding one drop. The drop did not yet reach the green foam. The second drop was allowed to drip directly into the green foam. This is shown by the last picture. It shows that the green foam becomes dark brown.
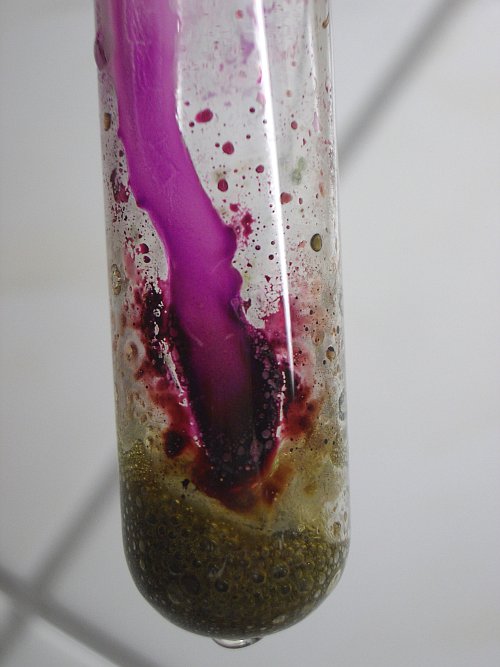


![]() Add much
more water to the mix.
Add much
more water to the mix.
When much more water is added, then the foam totally disappears and the liquid becomes dark purple brown. It looks like a solution of potassium permanganate, with a small part of it decomposed to hydrous manganese dioxide.

This picture nicely shows how much the glass is corroded along the path, where the first drop of water was going along the glass. A kind of jelly-like silica compound is formed, which is soaked full with a solution, containing the purple permanganate. On addition of an excess amount of some potassium metabisulfite, the permanganate is completely reduced to the colorless manganese (II) ion, but the purple streaks on the glass show that at that place the glass is heavily corroded and has a more spongy structure. The following large picture shows this effect.

A picture of the same test tube with a dark background shows the corrosion of the glass even more clearly.The liquid also is somewhat turbid. This is due to the presence of a suspension of SiO2 or acidic silica compounds.
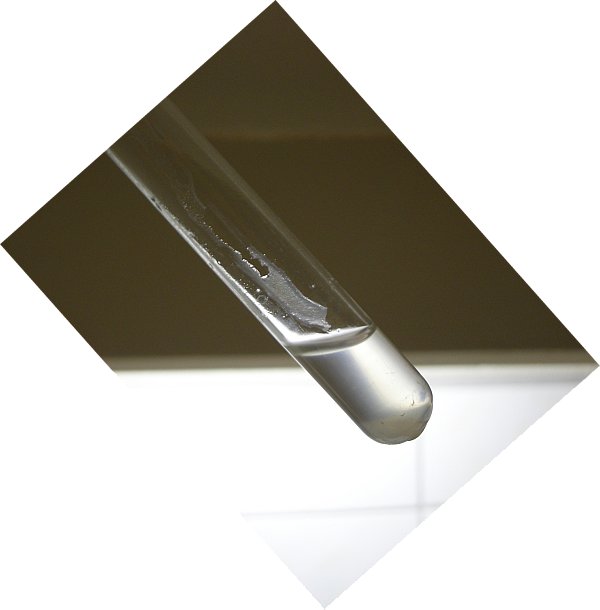
![]()
Discussion of results
![]() Excess sulphuric acid and sodium fluoride react as
follows:
Excess sulphuric acid and sodium fluoride react as
follows:
H2SO4 + NaF → HF + NaHSO4
This reaction is slow (much slower than the corresponding reaction of sulphuric acid with sodium chloride) and some heating is needed to drive off the gas.
![]() Permanganate reacts with sulphuric acid to form the compound Mn2O7,
which is dark brown/purple. With the fluoride, this, however, forms a green
compound. Literature mentions the existence of such a green compound (its vapors
also are said to be green, but in this experiment no colored vapors could be
observed). The constitution of this compound is not known for sure, at least not
by me. Probably it is a
compound with formula MnO3F, which is an oxo-fluoro-complex
of manganese (VII). Whatever the precise composition of the green compound, it
for sure contains manganese in the +7 oxidation state, because on addition of
water, the permanganate ion is formed back again. The compound also is volatile,
otherwise no reddish stains on the glass would form.
Permanganate reacts with sulphuric acid to form the compound Mn2O7,
which is dark brown/purple. With the fluoride, this, however, forms a green
compound. Literature mentions the existence of such a green compound (its vapors
also are said to be green, but in this experiment no colored vapors could be
observed). The constitution of this compound is not known for sure, at least not
by me. Probably it is a
compound with formula MnO3F, which is an oxo-fluoro-complex
of manganese (VII). Whatever the precise composition of the green compound, it
for sure contains manganese in the +7 oxidation state, because on addition of
water, the permanganate ion is formed back again. The compound also is volatile,
otherwise no reddish stains on the glass would form.
The hydrogen fluoride formed, especially in the presence of a small amount of water, is exceedingly corrosive. It quickly eats away the glass, as can be shown clearly by the pictures.