


Preparation of potassium chlorochromate(VI)
In this webpage it is described how an interesting compound of hexavalent chromium can be prepared, which is soluble in several organic solvents, allowing experimenting with chromium compounds in non-aqueous solution.
The starting material for this synthesis is potassium dichromate and good quality concentrated hydrochloric acid.

 Be
careful when performing this experiment. This experiment involves the handling
of warm highly concentrated solutions, containing hexavalent chromium. Such
solutions are very corrosive and toxic.
Be
careful when performing this experiment. This experiment involves the handling
of warm highly concentrated solutions, containing hexavalent chromium. Such
solutions are very corrosive and toxic.
Special care must be taken when handling the dry materials. Both potassium dichromate and the resulting compound potassium chlorochromate(VI) are non-hygroscopic solids which easily form dust. Especially the inhalation of dust of these compounds is harmful, because hexavalent chromium compounds are known carcinogens through this route of exposure.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium dichromate
-
concentrated hydrochloric acid (at least 25%) of good quality. The yellow/green material from a hardware store is not suitable and must be purified before use.
-
(dilute hydrochloric acid and sodium sulfite for safe disposal)
![]() Required
equipment:
Required
equipment:
-
wide test tube
- means of carefully heating the test tube (e.g. little alcohol burner)
- glass rod for stirring (metal is not suitable, some plastics may be suitable)
- sintered glass filter
![]() Safety:
Safety:
-
 Hexavalent
chromium is a known carcinogen when dust or droplets containing this are
inhaled. Exposure of the skin to hexavalent chromium compounds may lead to
allergic reactions.
Hexavalent
chromium is a known carcinogen when dust or droplets containing this are
inhaled. Exposure of the skin to hexavalent chromium compounds may lead to
allergic reactions. - Concentrated hydrochloric acid gives off nasty fumes and is very corrosive. The solutions, handled in this experiment are even more corrosive. If a drop of any of the solutions comes on the skin, then immediately rinse with water in which a small amount of sodium sulfite or bisulfite is dissolved, followed by a rinse with plain tap water.
![]() Disposal:
Disposal:
- Dissolve 2 grams of sodium (bi)sulfite in 20 ml of water, add a few ml of dilute hydrochloric acid and pour all waste liquid in this solution. Any hexavalent chromium then is reduced to the much less harmful trivalent chromium. The waste solutions then can be flushed down the drain, because only a few hundreds of mg of chromium waste is produced.
- Paper tissue waste, containing wet hexavalent chromium can be put in ordinary household waste. The hexavalent chromium quickly is reduced to trivalent chromium by the paper tissue.
![]()
Preparation of KCrO3Cl
The basis of this synthesis is the reaction between dichromate ion and concentrated hydrochloric acid. If the hydrochloric acid is of sufficient concentration, then not all HCl is dissociated into ions. Under those conditions one of the oxo-ligands in the dichromate ion can be replaced by a chloro-ligand. At lower concentration of the hydrochloric acid the reverse reaction occurs:
Cr2O72- + 2HCl ↔ 2CrO3Cl– + H2O
The potassium salt of this chlorochromate(VI) ion can be crystallized from the solution.
Because of the equilibrium reaction, the yield of this reaction never can be 100%. High yield is favored by increasing the concentration of the hydrochloric acid and also by increasing the concentration of potassium dichromate in the solution, such that more chlorochromate(VI) can be crystallized from the solution.
Based on above observations, when thinking naively, one would choose to dissolve as much as possible of potassium dichromate in acid of highest possible concentration, while assuring that excess HCl remains present. Unfortunately this does not lead to satisfactory results. There is a side reaction, in which hexavalent chromium oxidizes the hydrochloric acid, giving chlorine gas and trivalent chromium. This leads to an impure product. So, a good tradeoff has to be chosen between yield and purity.
Good results are obtained if following recipe is followed. Further below it is shown what happens if one is too greedy trying to obtain better yield. For good results with acceptable though not superb yield follow the procedure below.
![]() Put 1
gram of crushed potassium dichromate in a wide test tube. The material must be
crushed, big crystals certainly lead to less pure results due to slower
dissolving. The picture below shows potassium dichromate with crystalline pieces
not larger than 1 mm.
Put 1
gram of crushed potassium dichromate in a wide test tube. The material must be
crushed, big crystals certainly lead to less pure results due to slower
dissolving. The picture below shows potassium dichromate with crystalline pieces
not larger than 1 mm.

![]() Take 5
ml of 30% hydrochloric acid and mix this with 1 ml of distilled water, such that
approximately 25% hydrochloric acid is obtained. The acid must be pure and
colorless, do not use the yellow/green acid from a hardware store.
Take 5
ml of 30% hydrochloric acid and mix this with 1 ml of distilled water, such that
approximately 25% hydrochloric acid is obtained. The acid must be pure and
colorless, do not use the yellow/green acid from a hardware store.
![]() Pour the
25% acid in the test tube with the potassium dichromate. When this is done,
immediately some potassium dichromate dissolves, but the largest part simply
does not dissolve and forms a solid crust of material at the bottom of the test
tube.
Pour the
25% acid in the test tube with the potassium dichromate. When this is done,
immediately some potassium dichromate dissolves, but the largest part simply
does not dissolve and forms a solid crust of material at the bottom of the test
tube.

![]() Carefully heat the test tube with a small flame, while swirling it constantly.
Use a glass rod to stir the liquid in the test tube if the solid forms a hard
crust. It is of utmost importance that the heating is not too strong and that
no hot spots are formed under solid material. If the liquid becomes too hot,
then the hexavalent chromium oxidizes the hydrochloric acid and the experiment
can be regarded as failed. Keep on heating until all of the potassium dichromate
has dissolved and then immediately stop heating. The resulting liquid has
a beautiful deep red color.
Carefully heat the test tube with a small flame, while swirling it constantly.
Use a glass rod to stir the liquid in the test tube if the solid forms a hard
crust. It is of utmost importance that the heating is not too strong and that
no hot spots are formed under solid material. If the liquid becomes too hot,
then the hexavalent chromium oxidizes the hydrochloric acid and the experiment
can be regarded as failed. Keep on heating until all of the potassium dichromate
has dissolved and then immediately stop heating. The resulting liquid has
a beautiful deep red color.

![]() When all
potassium dichromate is dissolved, then set the test tube aside and let it cool
down. While it is cooling down, crystals are formed in the liquid.
When all
potassium dichromate is dissolved, then set the test tube aside and let it cool
down. While it is cooling down, crystals are formed in the liquid.
![]() The
yield can be increased by cooling down much further. When the test tube has
cooled down almost to room temperature, then put it in a jar, filled with ice
and snow.
The
yield can be increased by cooling down much further. When the test tube has
cooled down almost to room temperature, then put it in a jar, filled with ice
and snow.


This lowers the temperature to well below 0 ºC and when this is done additional solid material settles at the bottom of the test tube. The formation of the crystals looks very nice, glittering plates are formed in the liquid and these slowly sink to the bottom. A remarkably thick layer of crystalline solid is formed.

![]() Keep the liquid in the ice/snow mix for 15 minutes and then take out the test
tube and decant the liquid above the crystal mass. This decanting of liquid can
be done very easily, the crystal mass is compact. The test tube, with the liquid
decanted from the solid material has approximately half of its volume left. The
crystals are orange, not as deep red as the solution.
Keep the liquid in the ice/snow mix for 15 minutes and then take out the test
tube and decant the liquid above the crystal mass. This decanting of liquid can
be done very easily, the crystal mass is compact. The test tube, with the liquid
decanted from the solid material has approximately half of its volume left. The
crystals are orange, not as deep red as the solution.

![]()
Workup of the KCrO3Cl
Working up of the solid material is fairly easily done, but some special precautions must be taken, due to the strongly oxidizing properties of the material.
![]() Carefully tap the solid but still very wet material on a sintered glass filter.
Do not use normal filter paper, because that reacts with the material and spoils
the product!
Carefully tap the solid but still very wet material on a sintered glass filter.
Do not use normal filter paper, because that reacts with the material and spoils
the product!

Keep the material on the filter for a few minutes until no more liquid drips out of the filter.
![]() After a few minutes, take some paper tissue and press that on the bottom side of
the glass filter, such that liquid is absorbed from the glass filter. When the
paper tissue is wetted through, then repeat this action another time. For making
this web page, the construction was taken in the hand, the picture below shows
how the liquid can be absorbed into paper tissue.
After a few minutes, take some paper tissue and press that on the bottom side of
the glass filter, such that liquid is absorbed from the glass filter. When the
paper tissue is wetted through, then repeat this action another time. For making
this web page, the construction was taken in the hand, the picture below shows
how the liquid can be absorbed into paper tissue.
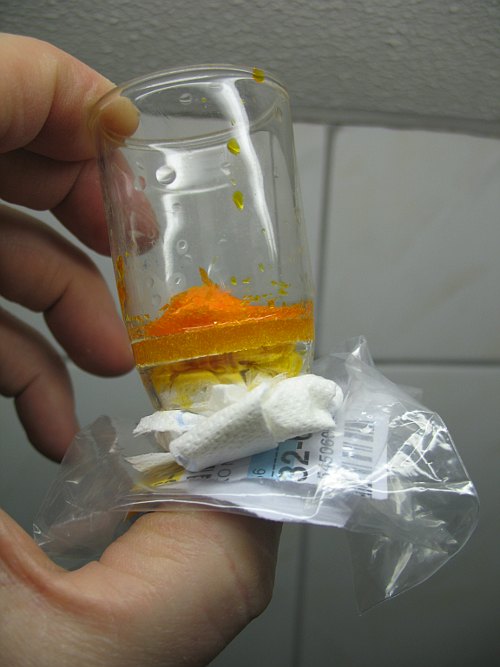
After two times of absorbing liquid into paper tissue, the orange solid is fairly dry. The tissue paper itself is soaked with orange liquid. After 15 minutes, however, the paper tissue is green. This nicely shows that the hexavalent chromium reacts with the paper tissue. The left picture was made immediately after absorbing the liquid in the paper, the right picture was made 15 minutes later.


![]() Scrape the solid from the glass filter and put it on a watch glass and allow the
solid to dry in a warm place. Do not really heat it, it must dry on a warm
place, such as on top of a heating radiator (at 40 ºC or so). When the solid is
heated, then it decomposes. The solid on the hour glass looks as follows:
Scrape the solid from the glass filter and put it on a watch glass and allow the
solid to dry in a warm place. Do not really heat it, it must dry on a warm
place, such as on top of a heating radiator (at 40 ºC or so). When the solid is
heated, then it decomposes. The solid on the hour glass looks as follows:

![]() After drying for a few hours, spread out the solid a little more and allow it to
dry for another few hours. Then transfer it to a clean and dry vial. The final
result is just over 0.5 grams of orange/red solid.
After drying for a few hours, spread out the solid a little more and allow it to
dry for another few hours. Then transfer it to a clean and dry vial. The final
result is just over 0.5 grams of orange/red solid.

This solid is pure potassium chlorochromate. This material dissolves in acetone exceptionally well, a spatula of solid dissolves in the acetone when it is poured over it, no shaking or heating is necessary at all. Such a solution is orange, just like a solution of potassium dichromate in water. When the solid is dissolved in water, then it is hydrolysed to potassium dichromate and hydrochloric acid. So, for experiments in aqueous solution this compound is not that interesting, but it is interesting for experiments in organic non-aqueous solvents.
When potassium chlorochromate is heated, then a colored gas mix is produced, which is yellow/brown in appearance. Probably the color of the gas mix is due to the mix of the color of chlorine and chromyl chloride. The color of the gas mix is not very strong, but it is clearly visible.

![]()
A failed attempt to increase yield
A second attempt was made to increase the yield. Instead of 1 gram, 1.5 grams of potassium dichromate was added to a mix of 5 ml of 30% hydrochloric acid and 1 ml of distilled water. The idea behind this experiment was that the additional 0.5 gram will lead to much more crystals from the same (still excess) amount of acid.
When this is done, then initially the experiment proceeds as described above. However, dissolving of the additional 0.5 grams of potassium dichromate requires much stronger heating. At a certain point, suddenly the color shifts from deep red to brown and bubbles of chlorine gas escape from the liquid. From that moment, even when heating is stopped immediately, the redox reaction continues, leading to a very dark brown liquid with still some solid potassium dichromate at the bottom. Before crystallization of potassium chlorochromate started, the dark liquid was poured in another test tube, otherwise the separation from the still not dissolved potassium dichromate would be very hard. In the other test tube, the crystallization started and a lot of crystalline material was obtained. The entire volume of liquid was filled with crystals, so indeed, the yield is better than with just 1.0 grams of potassium dichromate.


The material, isolated from the dark liquid is much less pure. It is brown instead of orange/red. About half the amount of solid material was transferred to a little vial after filtering and drying. The picture below nicely demonstrates the difference between the two products.

![]()
Discussion of results
![]() Hexavalent chromium coordinates well to oxygen, leading to formation of the
well-known chromate and dichromate ions. Under certain conditions, however,
oxo-ligands can be replaced by chloro-ligands. In aqueous concentrated
hydrochloric acid, one oxo-ligand of chromate can be replaced by chloride,
leading to formation of CrO3Cl–. Under more extreme
conditions, a second oxo-ligand can be replaced by chloride, leading to
formation of CrO2Cl2. This requires anhydrous conditions
in the presence of concentrated sulphuric acid.
Hexavalent chromium coordinates well to oxygen, leading to formation of the
well-known chromate and dichromate ions. Under certain conditions, however,
oxo-ligands can be replaced by chloro-ligands. In aqueous concentrated
hydrochloric acid, one oxo-ligand of chromate can be replaced by chloride,
leading to formation of CrO3Cl–. Under more extreme
conditions, a second oxo-ligand can be replaced by chloride, leading to
formation of CrO2Cl2. This requires anhydrous conditions
in the presence of concentrated sulphuric acid.
![]() In theory at 100% yield one would have the following
reaction:
In theory at 100% yield one would have the following
reaction:
K2Cr2O7 + 2HCl ↔ 2KCrO3Cl + H2O
From 1 gram of potassium dichromate, 1.187 gram of potassium chlorochromate(VI) could be obtained. In this experiment a little over 0.5 gram was obtained, which is a yield of 43%. The biggest loss is in the solubility of the salt in hydrochloric acid.
![]() Higher yield can be obtained by evaporating some of
the acid, increasing the concentration of the acid, or adding more potassium
dichromate, but all of these methods also lead to a side reaction, in which
trivalent chromium and chlorine are formed. The product then is brown instead of
orange/red. Purifying the product then can be done by recrystallizing from
concentrated hydrochloric acid, but this then leads to considerable losses,
undoing the initial increase of yield.
Higher yield can be obtained by evaporating some of
the acid, increasing the concentration of the acid, or adding more potassium
dichromate, but all of these methods also lead to a side reaction, in which
trivalent chromium and chlorine are formed. The product then is brown instead of
orange/red. Purifying the product then can be done by recrystallizing from
concentrated hydrochloric acid, but this then leads to considerable losses,
undoing the initial increase of yield.