


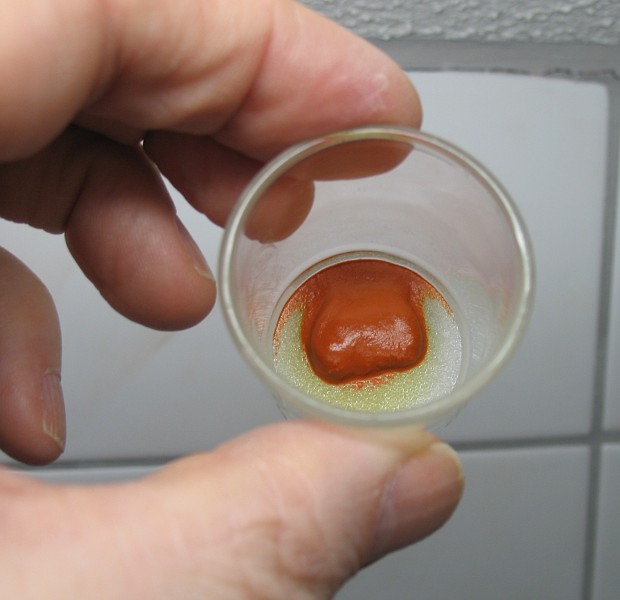
Red complex of copper and chloride
Compounds of copper(II) usually are blue, green and all kinds of color between these. Some complexes are olive-green, but all common complexes have colors are in the range of blue and green.
In this experiment, however, a remarkable complex is made with a bright orange/red color and it is even more remarkable that this complex is a complex of copper(II) and chloride. No special ligands are used for making the red color. A suitable stabilizing cation, however, is needed to allow the separation of this complex from its aqueous solution.

The picture above shows the red/orange color of the complex ion CuCl3–, precipitated as the cesium salt, CsCuCl3.
![]()
![]() Required
chemicals:
Required
chemicals:
-
cupric chloride dihydrate (the anhydrous compound also can be used)
-
concentrated hydrochloric acid (30% or more HCl by weight)
-
cesium chloride
-
potassium chloride
-
tetramethylammonium chloride (optional)
![]() Required
equipment:
Required
equipment:
-
test tubes
-
sintered glass filter
-
paper tissue
![]() Safety:
Safety:
-
Concentrated hydrochloric acid is very corrosive and gives nasty fumes. Avoid breathing the fumes. If some of the acid is spilled on the skin, then quickly rinse with water under a running tap.
![]() Disposal:
Disposal:
-
Copper salts are rather toxic for aquatic life (plant life and microbial life) and hence the waste should not be flushed down the drain. Neutralize the waste somewhat with a solution of sodium hydroxide and then bring it to a municipal waste processing facility.
![]()
Preparation of the solutions
![]() Take a spatula full of cupric chloride and put this in a
clean test tube. Add a small amount of concentrated hydrochloric
acid. Add so much that the solid just dissolves. Try to make the solution as
concentrated as possible.
Take a spatula full of cupric chloride and put this in a
clean test tube. Add a small amount of concentrated hydrochloric
acid. Add so much that the solid just dissolves. Try to make the solution as
concentrated as possible.
The top left picture below shows solid cupric chloride in a test tube. The other pictures show the situation after adding concentrated hydrochloric acid (30%) and dissolving all of the solid. This solution has a dark olive green color. The liquid shows intense absorption, such that even a thin layer, sticking to the glass, shows a strong yellow/green color.



![]() Put some cesium chloride in a test tube and add a small
amount of water. The resulting solution need not be totally saturated, but it
must be quite a concentrated solution. Below, a test tube is shown with the
cesium chloride and the solution of this in water.
Put some cesium chloride in a test tube and add a small
amount of water. The resulting solution need not be totally saturated, but it
must be quite a concentrated solution. Below, a test tube is shown with the
cesium chloride and the solution of this in water.


![]()
Formation of the complex
Making the complex is very simple. Just add the solution of cupric chloride to the solution of cesium chloride. When this is done, immediately a precipitate is formed. When there is excess cesium chloride, then a yellow precipitate is obtained, when there is excess cupric chloride, then a red precipitate is obtained.
The picture below shows the result of adding a single big drop of solution of cupric chloride to the solution of cesium chloride. As the picture shows, most of the precipitate is yellow, due to the excess amount of cesium chloride. Only at spots where there is a large concentration of cupric chloride the precipitate is red.

When all cupric chloride is added, then a really colorful mix is obtained. There still is green from the solution of cupric chloride, there is red under the green solution and yellow more deeply, where the concentration of cesium chloride is higher. The precipitate is compact, it quickly settles at the bottom. It is not a slimy precipitate, but microcrystalline. There is a slight excess of cupric chloride.

When the test tube is shaken, then all of it turns orange/red, there was an excess amount of cupric chloride.

![]()
Isolation of the complex
Isolating the complex is not really difficult if a sintered glass filter is used. The liquid with the precipitate must be poured slowly on the glass filter. The liquid quickly is absorbed by the filter and a fairly dry and compact heap of red/orange solid remains.
A piece of paper tissue can be pushed against the other side of the glass filter such that almost all of the liquid is absorbed. The remaining red/orange solid is fairly pure, although it still will contain some HCl and CuCl2.
The material can be dried on a warm place (e.g. a heat radiator). It does not dry well at room temperature. The color changes from red to yellow when the material dries. The picture below shows a dried sample and a sample, freshly taken from the liquid.

Both the dry and the wet material dissolve in water very well, giving a clear light blue solution. Dissolving of the solid is shown in a short video (download size appr. 1.2 MByte). The change of color is due to breakdown of the complex, see explanation at the end of this webpage.
![]()
Replacing cesium chloride by potassium chloride
The experiment was repeated with potassium chloride instead of cesium chloride. The solubility of potassium chloride in water is lower than that of cesium chloride, hence the solution of potassium chloride was heated somewhat (to 60 °C or so) in order to get a solution of high concentration.
When the solution of potassium chloride is mixed with the solution of cupric chloride in concentrated hydrochloric acid, then a totally different result is obtained. No yellow or red solid is formed, but a compact white crystalline solid is formed, which quickly settles at the bottom. The dark green solution of cupric chloride is diluted and shifts color somewhat from olive green to grass green.

The solid simply is potassium chloride. This is only very sparingly soluble in hydrochloric acid and it precipitates from the concentrated solution.
To this green liquid with white precipitate some solid cesium chloride was added. When this is done, immediately a red precipitate is formed and this slowly creeps into the white precipitate. It looks as if the cesium chloride dissolves in the acid and reacts with the solution of cupric chloride. Two minutes after adding the solid cesium chloride, the contents of the test tube looks as follows:

After shaking, all of the liquid becomes red/brown. The color is more dirty red/brown. This is due to the strong excess amount of cupric chloride, only a small amount of cesium chloride was added.

The color of the precipitate also is more pale. This also can be explained, because the solid only partially consists of the red complex, a considerable part is finely divided potassium chloride.
This experiment also was done with N(CH3)4Cl, which has a large cation. With this compound, however, no precipitate is formed at all. On mixing of the liquids, the resulting liquid becomes green and clear.
![]()
Results with other metals than copper
This experiment also was performed with cobaltous chloride and ferric chloride instead of cupric chloride.
When a solution of cesium chloride is mixed with a deep blue solution of cobaltous chloride in concentrated hydrochloric acid, then a very fine microcrystalline dull-blue precipitate is formed.

When a solution of cesium chloride is mixed with a yellow/brown solution of ferric chloride in concentrated hydrochloric acid, then a very fine microcrystalline yellow/orange precipitate is formed. The liquid above the precipitate also shifts color somewhat. It becomes lighter and shifts from yellow/brown to yellow/orange.

The blue and yellow/orange precipitate can be isolated like the precipitate from the copper salt. Both precipitates are soluble very well in water. When water is added to the two test tubes, such that the volume of liquid is roughly tripled, then the precipitates completely dissolve and in the case of cobalt, the association with the chloride also is lost (shift from blue to pink).

These experiments show that cesium also forms solid complexes with cobalt(II) and iron(II).
Similar experiments also were done with nickel chloride and chromium(III) chloride. These metals, however, do not lead to formation of solid complexes. No precipitate is formed on addition of cesium chloride to solutions of these metal chlorides in concentrated hydrochloric acid.
![]()
Discussion of results
Solid hydrated cupric chloride is not a classical salt like NaCl with clearly separated ions. Instead it can better be regarded as a neutral complex:
CuCl2(H2O)2
This complex has two chloride ions and two water molecules coordinated to a central copper ion.
![]() When cupric chloride is dissolved in concentrated
hydrochloric acid, then a mix of complexes is formed, with the equilibrium
strongly to the right, due to high chloride concentration:
When cupric chloride is dissolved in concentrated
hydrochloric acid, then a mix of complexes is formed, with the equilibrium
strongly to the right, due to high chloride concentration:
CuCl2(H2O)2 + Cl– → [CuCl3(H2O)]– + H2O (the complex ion [CuCl3(H2O)]– is green)
[CuCl3(H2O)]– + Cl– ↔ [CuCl4]2– + H2O (the complex ion [CuCl4]2– is dark green/brown)
The solution has a deep olive green color.
When a lot of water is added, then chloride ligands are replaced by water ligands, leading to the typical light blue color of aqueous copper(II) ion.
![]() On
addition of cesium ions, a very peculiar set of reactions occurs. The cesium salt of the
complex ion [CuCl3(H2O)]–
is very sparingly soluble in concentrated hydrochloric acid and precipitates
from this. While the cesium ion and the complex ion combine into a crystal
lattice, water molecules are split off and go into solution.
On
addition of cesium ions, a very peculiar set of reactions occurs. The cesium salt of the
complex ion [CuCl3(H2O)]–
is very sparingly soluble in concentrated hydrochloric acid and precipitates
from this. While the cesium ion and the complex ion combine into a crystal
lattice, water molecules are split off and go into solution.
Cs+ + [CuCl3(H2O)]– ↔ Cs[CuCl3] + H2O
Although the formula given in this equation is Cs[CuCl3], the structure is polymeric, so a better formula would be Csn[CuCl3]n with n being a very large number. The structure consists of tetrahedral CuCl3–-units, where the face with three Cl-atoms on its vertices is shared by two copper atoms. Hence, each copper atom is coordinated to 6 chlorine atoms in the chain. The negative charge of the chain is counterbalanced by the charge of the cesium cations. This solid has a garnet red color and when it is divided finely, then it appears orange/red.
![]() At high chloride concentration, most of the copper will be
present as [CuCl4]2– and when at the same time the cesium
concentration is high, then the yellow Cs2[CuCl4] is
formed. This explains why in excess concentrated solution of CsCl a yellow solid
is precipitated while in excess solution of CuCl2 a red solid is
precipitated.
At high chloride concentration, most of the copper will be
present as [CuCl4]2– and when at the same time the cesium
concentration is high, then the yellow Cs2[CuCl4] is
formed. This explains why in excess concentrated solution of CsCl a yellow solid
is precipitated while in excess solution of CuCl2 a red solid is
precipitated.
![]() When
this solid is added to water, then it quickly dissolves, the reverse reaction
occurs. But in water at low chloride concentration, the reaction goes further,
finally leading to loss of all chloride ions and complete exchange with water
molecules:
When
this solid is added to water, then it quickly dissolves, the reverse reaction
occurs. But in water at low chloride concentration, the reaction goes further,
finally leading to loss of all chloride ions and complete exchange with water
molecules:
Cs[CuCl3] + H2O ↔ Cs+ + [CuCl3(H2O)]–
[CuCl3(H2O)]– + H2O ↔ CuCl2(H2O)2 + Cl–
CuCl2(H2O)2 + 2H2O ↔ CuCl(H2O)4+ + Cl–
CuCl(H2O)4+ + 2H2O ↔ Cu(H2O)6+ + Cl–
So, when the solid is added to water, then it dissolves, giving a pale blue solution when a large amount of water is added. When a small amount of water is added, then a green/cyan solution is obtained.
![]() The
cesium ion is quite special in this situation. K[CuCl3]
does not precipitate from the concentrated acid solution (instead KCl
precipitates) and [N(CH3)4][CuCl3] also does not
precipitate from the concentrated solution (no precipitate is formed at all).
According to literature,
K[CuCl3] can be made, but this is more
difficult and requires careful evaporation of a dilute solution of KCl and CuCl2
over a strong dehydrating agent.
The
cesium ion is quite special in this situation. K[CuCl3]
does not precipitate from the concentrated acid solution (instead KCl
precipitates) and [N(CH3)4][CuCl3] also does not
precipitate from the concentrated solution (no precipitate is formed at all).
According to literature,
K[CuCl3] can be made, but this is more
difficult and requires careful evaporation of a dilute solution of KCl and CuCl2
over a strong dehydrating agent.
The information about the color and the structure of the Csn[CuCl3]n complex is from the book "The chemical elements and their compounds", volume 1, page 162, written by N.V. Sidgwick, 1951.
A more recent investigation also confirms the presence of a chain structure, albeit that in this renewed research the structure appears to have three chlorine atoms shared by two copper atoms. More information about that can be found in Journal of Inorganic Chemistry, Volume 5, nr. 2, 1966 in a paper titled "A Redetermination of the Crystal Structure of CsCuCl3", written by Albert W. Schlueter, Robert A . Jacobson, and Robert E . Rundle.