Vanadyl acetylacetonate - VO(acac)2

Vanadyl ion can easily coordinate to the so-called acac-anion and the resulting complex is neutral and as such easily dissolves in many organic apolar solvents.
Acetyl acetone has the following structure:
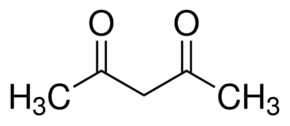
In aqueous solution, the compound easily forms tautomers and the following equilibrium occurs:
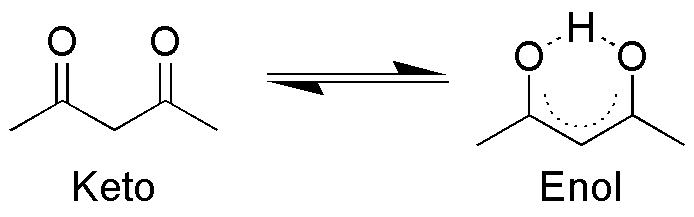
The enol form easily donates a H+
ion and then an anion, called acetylacetonate, also known as (acac)
remains. This ion, with two adjacent oxygen atoms, forms a very
strongly bidentately coordinating ion. One of the ions, easily
coordinated, is vanadyl. The picture above shows the complex in its
solid form, a blue/green compound, slightly soluble in water, much more
soluble in many organic solvents.