Copper acetate - (CH3COO)2Cu·H2O

Copper(II) acetate usually is available as the monohydrate, as a dark green/blue crystalline powder. The picture above shows how dark this is.
 With
a slightly overexposed flash-light image, the color is shown a little bit
better.
With
a slightly overexposed flash-light image, the color is shown a little bit
better.
The color of this compound differs a lot from the well-known blue or blue/cyan color of most other hydrated copper compounds. This dark color is due to the special nature of copper(II) acetate.
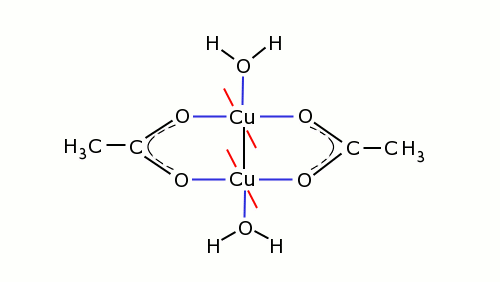
Copper(II) acetate forms a coordination complex of quite a remarkable structure. A better formula for copper(II) acetate would be (CH3COO)4Cu2(H2O)2. The compound has two directly connected copper atoms, which form a Cu2-center, and to this center, the four acetate-ligands and the two water ligands are connected. The structure is shown in the picture below. The black lines are normal bonds, the blue lines are coordinate bonds and the image shows that each acetate ion is connected to two copper atoms. The red lines are connected to two other acetate ions. These are not drawn in the picture, they must be thought of as being perpendicular to the plane of drawing. So, the four acetate ligands are around the two copper atoms. The two water molecules are above and below the line, setup by the two copper atoms.